2024/2025 Öğretim Yılı Güz Yarıyılı Ders Programı
2024/2025 Öğretim Yılı Güz Yarıyılı Ders Programı MBG BST
By loading the map, you agree to Google's privacy policy.
Learn more
| İzmir Uluslararası Biyotıp ve Genom Enstitüsü (iBG-izmir) Dokuz Eylül Üniversitesi Sağlık Yerleşkesi Balçova 35340 İzmir |
Telefon: 0(232) 412 86 51 Faks: 0(232) 412 63 53 E-posta: ibg@deu.edu.tr |
Enstitü Sekreterliği
| Enstitü Sekreteri V. | Salih AKAGÜNDÜZ | +90 232 412 86 54 | salih.akagunduz@deu.edu.tr |
| Özel Kalem | Ayşe Gül ÖZCAN | +90 232 412 86 51 | gul.ozcan@deu.edu.tr |
İdari Birimler
| Öğrenci İşleri | Gamze Güven | +90 232 412 86 66 | gamze.guven@deu.edu.tr |
| Yazı İşleri ve Evrak Kayıt Birimi | Özlem Alku | +90 232 412 86 70 | ozlem.alku@deu.edu.tr |
| Personel İşleri/Bölüm Sekreterliği | Naciye Yıldız | +90 232 412 86 63 | naciye.yildiz@deu.edu.tr |
| Dış İlişkiler Birimi | Ayşegül Özcan | +90 232 412 86 51 | gul.ozcan@deu.edu.tr |
| İdari ve Mali İşler Birimi | Haktan Güner | +90 232 412 86 61 | haktan.guner@deu.edu.tr |
| Taşınır Kayıt ve Kontrol Birimi | Haktan Güner | +90 232 412 86 61 | haktan.guner@deu.edu.tr |
Number Of Academic And Administrative Staff Of Izmir International Institute Of Biotype And Genome
Izmir International Institute Of Biotype And Genome Student Number ( Last 6 Years )
| Graduation Year | Graduate Programme | Doctorate | Integrated Doctor | Total |
| 2018 | 2 | 2 | ||
| 2019 | 22 | 22 | ||
| 2020 | 17 | 2 | 19 | |
| 2021 | 9 | 3 | 12 | |
| 2022 | 19 | 11 | 30 | |
| 2023 | 6 | 6 | 2 | 14 |
| 2024 |
Anabilim Dalı Başkanıhttp://debis.deu.edu.tr/akademiktr/index.php?cat=3&akod=20140107
Anabilim Dalı Akademik Personel Listesi
Moleküler Biyoloji ve Genetik Yüksek Lisans Programı
Moleküler Biyoloji ve Genetik Doktora Programı
Enstitü Tanıtım videosu bu sekmeden ulaşabilirsiniz.
THESIS PROPOSAL/TITLE/CONTENT CHANGE FORM
DOCTORAL PROFICIENCY EXAM PhD THESIS MONITORING COMMITTEE FORM PhD THESIS MONITORING COMMITTEE REPORT
THESIS MONITORING COMMITTEE ESTABLISHMENT FORM
THESIS MONITORING COMMITTEE OFFICIAL REPORT
MASTER’S THESIS DEFENSE EXAM REPORT
MASTER’S THESIS DEFENSE APPLICATION FORM
THESIS PROPOSAL/TITLE/CONTENT CHANGE FORM
İzmir Uluslararası Biyotıp Ve Genom Enstitüsü Yatay Geçiş Öğrenci Kabul Koşulları
ÖĞRENCİ İŞLERİ
Graduation certificate request form
Guest student application form
Suspension of the studies form
APPLICATION AND ADMISSION PROCESS TO THE MASTER’S PROGRAM
kjkjlk
Guest Student Applıcatıon Form
4- TURNITIN Plagiarism Program User Guide
5-Thesis Plagiarism Analysis Program
Click on the button to load the content from docs.google.com.
| Prof. Dr. İbrahim Mehmet Ali ÖKTEM | Director |
| Assoc. Prof. Şerif ŞENTÜRK | Vice Director |
| Assoc. Prof. Hasan Buğra ÇOBAN | Vice Director |
| Assoc. Prof. Serhat TOZBURUN | Member |
| Assistant ProfessorZeynep Ahsen KOÇER | Member |
| Prof. Dr. İbrahim Mehmet Ali ÖKTEM | Institute Director | Chairman of the Commission |
| Prof. Dr. Şerif ŞENTÜRK | Institute Vice Director | Member |
| Assoc. Prof. Hasan Buğra ÇOBAN | Institute Vice Director | Member |
| Mukaddes AKKEÇELİ | Institute Secretary | Member |
| Naciye YILDIZ | Human Resources | Member |
| NAME SURNAME | POSITION | AUTHORITY | CONTACT | |
| Prof. Dr. İbrahim Mehmet Ali ÖKTEM | Institute Director | Unit Risk Management Coordinator | 412 8650 | ali.oktem@deu.edu.tr |
| Prof. Dr. Şerif ŞENTÜRK |
Institute Vice Director
|
Risk Management Unit | 412 8653 | serif.senturk@deu.edu.tr |
| Assoc. Prof. Hasan Buğra ÇOBAN |
Institute Vice Director
|
Risk Management Unit | 412 8652 | bugra.coban@deu.edu.tr |
| Mukaddes AKKEÇELİ | Institute Secretary | Risk Management Unit | 412 8654 | mukaddes.akkeceli@deu.edu.tr |
| Naciye YILDIZ | Human Resources | Risk Management Unit | 412 8663 | naciye.yildiz@deu.edu.tr |
Mission and Vision
Our mission is to train distinguished, talented scientists and academics to become essential parts of the international health community. We will organize educational activities to share knowledge and experience, to develop technology and tools to resolve global health problems, and to improve human welfare. Additionally, we will form partnerships with other national and international research centers to become an innovative training center.
Our vision is to become an internationally respected, nationally prestigious institute, able to introduce countless scientific discoveries, and to become a pioneering education and research center that translates novel findings in the life sciences to benefit society’s health and welfare.
Enstitümüz web sayfasından “Özel Öğrenci Başvuru Formu”nu alınız.
Özel Öğrenci Başvuru Formunun Enstitü web sayfasında Akademik Takvimde ilan edilen tarihlerde Enstitümüze teslim edilmesi gerekmektedir.
“Özel Öğrenci” olarak almaya karar verdiğiniz ders/derslerin, koordinatörü ve Anabilim Dalı Başkanı ile görüşerek derse katılma isteğinizi bildiriniz.
Özel Öğrenci Başvuru formunu doldurarak imzaları tamamlayınız.
Onaylattığınız “Özel Öğrenci Başvuru Formu”, “Kimlik Fotokopisi”, “Öğrenci Belgesi”, “İngilizce Yeterlik Belgesinin Resmi Onaylı Örneği”ni teslim ediniz.
Özel Öğrenci Başvuru Formunun eksiksiz olarak doldurulması ve istenilen belgelerin eksiksiz Enstitü web sayfasında Akademik Takvimde ilan edilen tarihler arasında Enstitüye teslim edilmesi gerekmektedir.
Belirtilen tarihler arasında başvurular yapılmadığı takdirde başvurular kabul edilmeyecektir
Hizmet Damgalı Pasaport Talep Formu
Özel Öğrenci Başvuru Formu
Mezuniyet Belgesi Talep Dilekçesi
Başka kurumdan/Diğer Anabilim Dalından Almak İstenen Ders İle İlgili Dilekçe
PERSONEL İŞLERİ
Rektörlüğe Gönderilecek 39. Görevlendirme Formu
Görevlendirme Talep Formu (TR)
Görevlendirme Talep Formu (İNG)
IBG_PhD_Danışman Degisikligi Formu
IBG_PhD_II. Tez Danısmanı Atama Formu
IBG_PhD_Doktora Yeterlik Sınavı Basvuru Formu
IBG_PhD_Doktora Yeterlik Sonuc Degerlendirme Tutanak Formu
IBG_MSc/PhD_Tez Önerisi Baslıgı-Icerigi Degisiklik Formu
IBG_PhD_Tez Izleme Komitesi Olusturma
IBG_PhD_Tez Izleme Komite Uyeleri Degistirme Formu
IBG_PhD_Tez Izleme Raporu Formu
IBG_PhD_Tez Izleme Komitesi Tutanagı
IBG_PhD_Tez Savunma Basvuru Formu
IBG_PhD_Tez Savunma Sinavi Tutanagı
IBG_MSc/PhD_Tez Degerlendirme Formu
IBG_MSc_Danışman Degisikligi Formu
IBG_MSc_II. Tez Danısmanı Atama Formu
IBG_MSc/PhD_Tez Önerisi Baslıgı-Icerigi Degisiklik Formu
IBG_MSc_Tez Savunma Basvuru Formu
IBG_MSc_Tez Savunma Sınavı Tutanagı
IBG_MSc_Cevrim İci Tez Savunma Sınavı Tutanagı
IBG_MSc/PhD_Tez Degerlendirme Formu
Doktora Başvuru, Kayıt, Eğitim Süreci
**Doktora Mezuniyet Makalesi Niteliklerine İlişkin Enstitü Kurulu Kararı için tıklayınız.
Yüksek Lisans Başvuru, Kayıt, Eğitim Süreci
Moleküler Biyoloji ve Genetik Yüksek Lisans Programı
Moleküler Biyoloji ve Genetik Doktora Programı
Moleküler Biyoloji ve Genetik Bütünleşik Doktora Programı
Department Of Genomics And Molecular Biotechnology
Council of Higher Education, Graduate Training Regulation
IZMIR INTERNATIONAL BIOMEDICINE AND GENOME INSTITUTE ACADEMIC AND ADMINISTRATIVE STAFF NUMBERS
İZMİR ULUSLARARASI BİYOTIP VE GENOM ENSTİTÜSÜ ÖĞRENCİ SAYILARI ( SON 2 YIL)
| AKADEMIC YEAR | MSc | PhD | Total |
| 2016-2017 | 24 | 16 | 40 |
| 2017-2018 | 21 | 37 | 58 |
https://ibg.deu.edu.tr/wp-content/uploads/2018/07/yonetim-semasi.pdf
DEÜ Bilimsel Araştırma Projeleri
YÖK Bilimsel Araştırma ve Yayın Etiği Yönergesi
Yurtdışı Yükseköğretim Diploma ve Denkliği Yönetmeliği
YÖK Lisansüstü ve Öğretim Yönetmeliği
DEÜ Muafiyet ve İntibak Yönergesi
DEÜ Akademik Danışmanlık Yönergesi
DEÜ Lisansüstü Eğitim ve Öğretim Yönetmeliği
2020-2021 Öğretim Yılı Güz Yarıyılı Akademik Takvim
2020-2021 Öğretim Yılı Bahar Yarıyılı Akademik Takvimi
Misyonumuz, sağlık alanında uluslararası camiada etkin olabilecek seçkin, yetenekli bilim insanları ve akademisyenler yetiştirmek, insanlığın refah düzeyini arttırmak ve küresel sağlık sorunlarını azaltmaya yönelik bilgi, deneyim, teknoloji ve araç geliştirmeye yönelik eğitim faaliyetleri düzenlemek, ulusal ve küresel düzeyde eğitim ve araştırma paydaşları ile ortaklıklar oluşturarak inovatif bir eğitim merkezi olmaktır.
Vizyon
Ülkemizde en çok tercih edilen, dünya çapında saygın, uluslararası standartta, yaşam bilimlerindeki yeni bilimsel bilgiyi toplumun sağlık ve refahı için kullanılabilir hale dönüştüren, yaptığı bilimsel keşifler ile dünya çapında sağlık ve bilim politikalarına yön veren bir eğitim ve araştırma kurumu olmaktır.

By loading the map, you agree to Google's privacy policy.
Learn more
| Izmir International Biomedicine and Genome Institute (iBG-izmir) Dokuz Eylül University Saglik Yerleskesi Balcova 35340 Izmir/TURKEY |
Phone: 0(232) 412 67 04 Fax: 0(232) 277 63 53 E-mail: ibg@deu.edu.tr |
Enstitü Sekreterliği
| ACTING SECRETARY OF GRADUATE SCHOOL | Salih AKAGÜNDÜZ | +90 232 412 86 54 | salih.akagunduz@deu.edu.tr |
| EXECUTIVE ASSISTANT | Ayşe Gül ÖZCAN | +90 232 412 86 51 | gul.ozcan@deu.edu.tr |
İdari Birimler
| STUDENT AFFAIRS | Gamze Güven | +90 232 412 86 66 | gamze.guven@deu.edu.tr |
| SECRETARY OF THE DEPARTMENTS | Özlem Alku | +90 232 412 86 70 | ozlem.alku@deu.edu.tr |
| HUMAN RESOURCES | Naciye Yıldız | +90 232 412 86 63 | naciye.yildiz@deu.edu.tr |
| FOREIGN RELATIONS UNIT | Ayşegül Özcan | +90 232 412 86 51 | gul.ozcan@deu.edu.tr |
| FINANCE OFFICE | Haktan Güner | +90 232 412 86 61 | haktan.guner@deu.edu.tr |
İZMİR ULUSLARARASI BİYOTIP VE GENOM ENSTİTÜSÜ LİSANSÜSTÜ EĞİTİM PROGRAMLARI
Genom Bilimleri ve Moleküler Biyoteknoloji Anabilim Dalı
Moleküler Biyoloji ve Genetik Yüksek Lisans Programı
Moleküler Biyoloji ve Genetik Doktora Programı
Moleküler Biyoloji ve Genetik Bütünleşik Doktora Programı
LİSANSÜSTÜ PROGRAMLARA BAŞVURU
YUKSEK LISANS
DOKTORA
BÜTÜNLEŞİK DOKTORA
YABANCI ÖĞRENCİ BİLGİLENDİRME FORMU (ÖĞRENİM VİZESİ VE İKAMETGÂH TEZKERESİ ALABİLMEK ICİN YAPILACAK ISLEMLER)
| Prof. Dr. İbrahim Mehmet Ali ÖKTEM | Enstitü Müdürü | Komisyon Başkanı |
| Prof. Dr. Şerif ŞENTÜRK | Enstitü Müdür Yardımcısı | Üye |
| Doç. Dr. Hasan Buğra ÇOBAN | Enstitü Müdür Yardımcısı | Üye |
| Mukaddes AKKEÇELİ | Enstitü Sekreteri | Üye |
| Naciye YILDIZ | Bilgisayar İşletmeni | Üye |
| AD SOYAD | GÖREV | YETKİ | İLETİŞİM | E-POSTA |
| Prof. Dr. İbrahim Mehmet Ali ÖKTEM | Enstitü Müdürü | Birim Risk Koordinatörü | 412 8650 | ali.oktem@deu.edu.tr |
| Prof. Dr. Şerif ŞENTÜRK | Enstitü Müdür Yardımcısı | Birim Risk Yönetim Ekibi | 412 8653 | serif.senturk@deu.edu.tr |
| Doç. Dr. Hasan Buğra ÇOBAN | Enstitü Müdür Yardımcısı | Birim Risk Yönetim Ekibi | 412 8652 | bugra.coban@deu.edu.tr |
| Mukaddes AKKEÇELİ | Enstitü Sekreteri | Birim Risk Yönetim Ekibi | 412 8654 | mukaddes.akkeceli@deu.edu.tr |
| Naciye YILDIZ | Personel İşleri | Birim Risk Yönetim Ekibi | 412 8663 | naciye.yildiz@deu.edu.tr |
| Prof. Dr. İbrahim Mehmet Ali ÖKTEM | Enstitü Müdürü |
| Prof. Dr. Şerif ŞENTÜRK | Enstitü Müdür Yardımcısı |
| Doç. Dr. Hasan Buğra ÇOBAN | Enstitü Müdür Yardımcısı |
| Doç. Dr. Serhat TOZBURUN | Ana Bilim Dalı Başkanı – Üye |
| Dr. Öğr. Üyesi Zeynep Ahsen KOÇER | Ana Bilim Dalı Başkanı – Üye |
Dokuz Eylül Üniversitesi Teknoparkı (DEPARK) ve Teknoloji Transfer Ofisi (DETTO) yürütücülüğünde; İzmir Biyotıp ve Genom Merkezi (İBG), ATABAY Kimya Sanayi ve Ticaret A. Ş., Vestel A. Ş. ve Voltpro Elektrik Motorları paydaşlığında yürütülen TÜBİTAK 1812 BİGG Projesi “BİGGSİNERJI” ön başvuruları başlamıştır. Üniversitemiz öğretim elemanları ile ön lisans, lisans, yüksek lisans ve doktora öğrencileri, www.biggsinerji.com adresinden […]
Prof.Dr.Petek KORKUSUZ Hematopoetik ve Spermatogonyal Kök Hücre Nişlerinin Rejeneratif Tıp Bakış Açısıyla Karşılaştırılması
Üniversitemiz Rektör Yardımcısı Prof. Dr. Uğur Malayoğlu’nun katılımıyla gerçekleştirilen Endüstri 4.0 Koordinatörlüğü tarafından 19 Nisan 2019 tarihinde yapılan toplantıya Enstitümüz ev sahipliği yaptı.
YÖK 100/2000 Doktora Bursu Almaya Hak Kazanan Adaylar aşağıda listelenmiştir. 14/02/2018 saat 17:00’ye kadar Enstitümüze bizzat gelerek ya da bir yakınına vekalet vererek kayıtlarını yaptırmalıdırlar. Esra ESMERAY Aykut KURUOĞLU Işıl TAKAN Murat Caner YARIMÇAM Didem ÖKMEN Bora TAŞTAN Nazlı Eda KALELİ ÇOBANOĞLU Tevfik HATİPOĞLU Bengisu ULUATA DAYANÇ Merve ARSLAN Ayşe Yağmur AZBAZDAR Özlem Şilan COŞKUN […]
100/2000 YÖK Doktora Bursu kapsamında 2017/2018 BAHAR yarıyılında aşağıda belirtilen koşulları taşıyan adaylar arasından, aşağıda belirtilen alanlarda doktora öğrencisi alınacaktır. 100/2000 YÖK Doktora Bursu kapsamında aşağıda listelenen Doktora Programları ve alanlar için belirtilen sayıda doktora öğrencisi alınacaktır. Bu kapsamda eğitimine devam eden doktora öğrencilerine azami 4 yıl boyunca, bütünleşik doktora öğrencilerine azami 5 yıl boyunca […]
İngilizce Moleküler Biyoloji ve Genetik programlarımıza kayıt hakkı kazananlar isim sırasına göre aşağıda listelenmiştir. Yüksek Lisans Çağla AKMAN Cihan Uğur OTÇU Kaan OKAY Muhammet Ekin AZBAZDAR Ozan YETİŞ Özge ÇARK Doktora Ayşe Yağmur AZBAZDAR Bengisu ULUATA DAYANÇ Bora TAŞTAN Burcu EKİNCİ Defne ENGÜR Elif KAYA Merve ARSLAN Murat KARADAĞ Nazlı Eda KALELİ Özlem Şilan COŞKUN […]
Yüksek Lisans Adayları (24/01/2018 – 09:00’dan itibaren) ADI SOYADI 1 Ayşe MEMON 2 Barış BEKTAŞ 3 Bekir Cem KUŞDEMİR 4 Cansu AZAK 5 Çağla AKMAN 6 Çağlar ÇELEBİ 7 Çiğdem BOZ 8 Cihan Civan CİVAŞ 9 Cihan Uğur OTÇU 10 Esmanur EREN 11 Hüseyin Koray MISIRLIOĞLU 12 Kaan OKAY Yüksek Lisans Adayları (24/01/2018 – 13:00’ten […]
6550 sayılı Araştırma Altyapılarının Desteklenmesine Dair Kanun kapsamında İzmir Biyotıp ve Genom Merkezi (İBG)’ne Araştırma Altyapıları Kurulu’nun 16/07/2017 tarihli ve 2017/1 sayılı kararı uyarınca yeterlik verilmiştir. Böylelikle İBG “Tematik Araştırma Altyapısı” olarak tüzel kişilik kazanmıştır.
Variant Filtering and Privacy in Genomic Data Speaker Mete AKGÜN, Ph.D. TUBITAK BILGEM, Turkey Date & Time January 18, 2018 13:30 Venue Dokuz Eylul University iBG-izmir Aziz Sancar Auditorium Inciraltı, IZMIR About the speaker Mete Akgün is a Chief Researcher at TÜBİTAK-BİLGEM, Kocaeli, Turkey. He received his M.S. and Ph.D. degrees from Department of Computer […]
Discovery and genotyping of novel sequence insertions in many sequenced individuals Speaker Pınar KAVAK, Ph.D. TUBITAK BILGEM, Turkey Date & Time January 18, 2018 13:00 Venue Dokuz Eylul University iBG-izmir Aziz Sancar Auditorium Inciraltı, IZMIR About the speaker Dr. Pınar Kavak has got her B.Sc. degree from Bilkent University, Department of Computer Engineering in […]
İZMİR ULUSLARARASI BİYOTIP VE GENOM ENSTİTÜSÜ AKADEMİK VE İDARİ PERSONEL SAYILARI
İZMİR ULUSLARARASI BİYOTIP VE GENOM ENSTİTÜSÜ ÖĞRENCİ SAYILARI ( SON 6 YIL)
| MEZUNİYET YILI | YÜKSEK LİSANS | DOKTORA | BÜTÜNLEŞİK DOKTORA | TOPLAM |
| 2018 | 2 | 2 | ||
| 2019 | 22 | 22 | ||
| 2020 | 17 | 2 | 19 | |
| 2021 | 9 | 3 | 12 | |
| 2022 | 19 | 11 | 30 | |
| 2023 | 13 | 11 | 2 | 26 |
| 2024 | 4 | 6 | 10 |
| athanasia.pavlopoulou@deu.edu.tr |
OVERVIEW
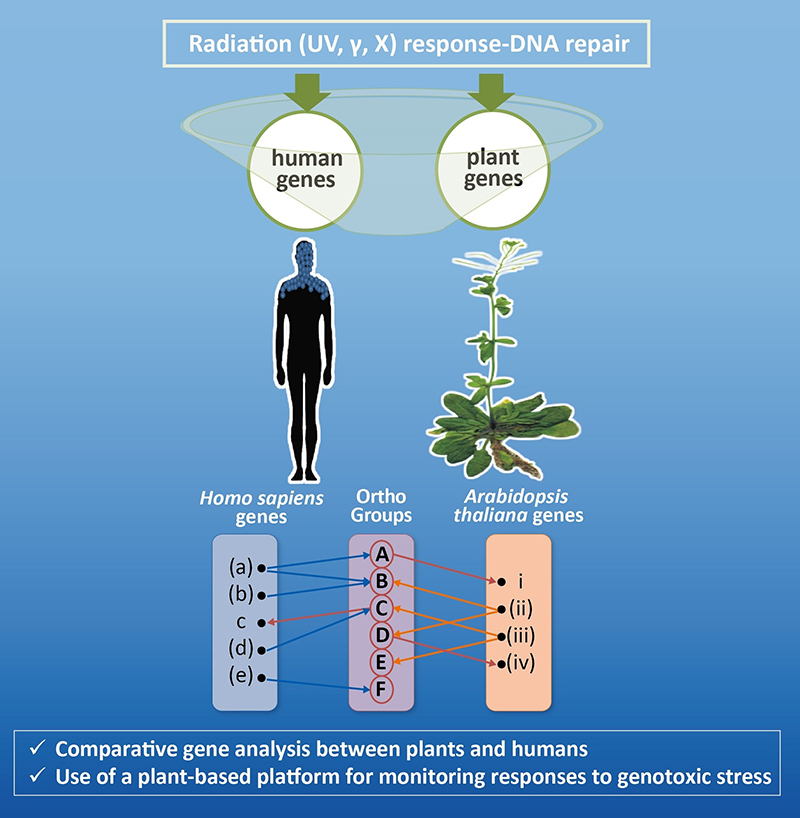
In the post-genomics era, there is undoubtedly a plethora of accumulated genomic, genetic and proteomic data. However, there is a growing gap between the biological molecules (DNA, RNA and proteins) with experimentally determined function/structure and the ones that have not been assigned to any functional/structural annotation. To handle this overwhelming amount of information, we apply bioinformatics and systems biology methods for processing, analyzing, and interpreting biological data in a meaningful way.
RESEARCH INTERESTS
My main research interests include:
Lab Members
Pavlopoulou lab is currently seeking highly motivated graduate students interested in biostatistics. If interested, please contact athanasia.pavlopoulou@deu.edu.tr
| PUBLICATIONS (selected) Full list and citations can be found at Pubmed and Google Scholar: “Athanasia Pavlopoulou” • Nikitaki Z, Holá M, Donà M, Pavlopoulou A, Michalopoulos I, Angelis KJ, Georgakilas AG, Macovei A, Balestrazzi A (2018) Integrating plant and animal biology for the search of novel DNA damage biomarkers. Mutation Research/Reviews in Mutation Research, 775: 21-38 • Pavlopoulou A* (2018) RecA: a universal drug target in pathogenic bacteria. Frontiers in Bioscience, 23: 36-42 • Geronikolou SA, Pavlopoulou A, Kanaka-Gantenbein C, Chrousos G (2018) Inter-species functional interactome of nuclear steroid receptors (R1). Frontiers in Bioscience, 10: 208-228 • Geronikolou SA, Pavlopoulou A, Cokkinos D, Chrousos G (2017) Interactome of Obesity: Obesidome. Advances in Experimental Medicine and Biology, 987: 233-241 • Pavlopoulou A*, Bagos PG, Koutsandrea V, Georgakilas AG (2017) Molecular determinants of radiosensitivity in normal and tumor tissue: A bioinformatic approach. Cancer Letters, 403: 37-47 • Nikitaki Z, Pavlopoulou A, Holá M, Donà M, Michalopoulos I, Balestrazzi A, Angelis KJ, Georgakilas AG (2017) Bridging Plant and Human Radiation Response and DNA Repair through an In Silico Approach. Cancers, 9: 6 • Dimitriou NM, Tsekenis G, Balanikas EC, Pavlopoulou A, Mitsiogianni M, Mantso T, Pashos G, Boudouvis AG, Lykakis IN, Tsigaridas G, Panayiotidis MI, Yannopapas V, Georgakilas AG (2017) Gold nanoparticles, radiations and the immune system: Current insights into the physical mechanisms and the biological interactions of this new alliance towards cancer therapy. Pharmacology & Therapeutics, 178: 1-17 • Galtsidis S, Logotheti S, Pavlopoulou A, Zampetidis CP, Papachristopoulou G, Scorilas A, Vojtesek B, Gorgoulis V, Zoumpourlis V (2017) Unravelling a p73-regulated network: The role of a novel p73-dependent target, MIR3158, in cancer cell migration and invasiveness. Cancer Letters, 388: 96-106 • Kontou PI#, Pavlopoulou A#, Dimou NL, Pavlopoulos GA, Bagos PG (2016) Data and programs in support of network analysis of genes and their association with diseases. Data in Brief, 8: 1036-1039 • Kontou PI#, Pavlopoulou A#, Dimou NL, Pavlopoulos GA, Bagos PG (2016) Network analysis of genes and their association with diseases. Gene, 590: 68-78 • Pavlopoulou A*, Oktay Y, Vougas K, Louka M, Vorgias CE, Georgakilas AG (2016) Determinants of resistance to chemotherapy and ionizing radiation in breast cancer stem cells. Cancer Letters, 380: 485-493 • Pavlopoulou A, Savva GD, Louka M, Bagos PG, Vorgias CE, Michalopoulos I, Georgakilas AG (2016) Unraveling the mechanisms of extreme radioresistance in prokaryotes: Lessons from nature. Mutation Research/Reviews in Mutation Research, 767: 92-107 • Skourti E, Logotheti S, Kontos CK, Pavlopoulou A, Dimoragka PT, Trougakos IP, Gorgoulis V, Scorilas A, Michalopoulos I, Zoumpourlis V. (2016) Progression of mouse skin carcinogenesis is associated with the orchestrated deregulation of mir-200 family members, mir-205 and their common targets. Molecular Carcinogenesis, 55: 1229-1242 • Pavlopoulou A, Scorilas A (2014) A comprehensive phylogenetic and structural analysis of the carcinoembryonic antigen (CEA) gene family. Genome Biology and Evolution 6: 1314–1326 • Vlachakis D, Pavlopoulou A, Roubelakis MG, Feidakis C, Anagnou NP, Kossida S (2014) 3D molecular modeling and evolutionary study of the Trypanosoma brucei DNA Topoisomerase IB, as a new emerging pharmacological target. Genomics, 103: 107-113 • Georgakilas AG#, Pavlopoulou A#, Louka M, Nikitaki Z, Vorgias CE, Bagos PG, Michalopoulos I (2015) Emerging molecular networks common in ionizing radiation, immune and inflammatory responses by employing bioinformatics approaches. Cancer Letters, 368: 164-172 • Pavlopoulou A, Spandidos DA, Michalopoulos I (2015) Human cancer databases (review). Oncology Reports, 33: 3-18 • Pavlopoulou A, Vlachakis D, Balatsos NA, Kossida S (2013) A comprehensive phylogenetic analysis of deadenylases. Evolutionary Bioinformatics Online, 9: 491-497 • Logotheti S, Pavlopoulou A, Galtsidis S, Vojtesek B, Zoumpourlis V (2013) Functions, divergence and clinical value of TAp73 isoforms in cancer. Cancer and Metastasis Reviews, 32: 511-534 • Vlachakis D, Pavlopoulou A, Kazazi D, Kossida S (2013) Unraveling microalgal molecular interactions using evolutionary and structural bioinformatics. Gene, 528: 109-119 • Vlachakis D, Tsiliki G, Pavlopoulou A, Roubelakis MG, Tsaniras SC, Kossida S (2013) Antiviral Stratagems Against HIV-1 Using RNA Interference (RNAi) Technology. Evolutionary Bioinformatics Online, 9: 203-213 • Vlachakis D, Pavlopoulou A, Tsiliki G, Komiotis D, Stathopoulos C, Balatsos NA, Kossida S (2012) An integrated in silico approach to design specific inhibitors targeting human poly(a)-specific ribonuclease. PLoS One, 7: e51113 • Attwood TK, Coletta A, Muirhead G, Pavlopoulou A, Philippou PB, Popov I, Romá-Mateo C, Theodosiou A, Mitchell AL (2012) The PRINTS database: a fine-grained protein sequence annotation and analysis resource–its status in 2012. Database (Oxford), 2012: bas019 • Pavlopoulou A, Michalopoulos I. (2011) State-of-the-art bioinformatics protein structure prediction tools (Review). International Journal of Molecular Medicine, 28: 295-310 • Pavlopoulou A, Kossida S (2010) Cytosine methyltransferases as tumor markers. Current Genomics 11: 568-577 • Pavlopoulou A, Pampalakis G, Michalopoulos I, Sotiropoulou G (2010) Evolutionary history of tissue kallikreins. PLoS One, 5: e13781 • Pavlopoulou A, Kossida S (2009) Phylogenetic analysis of the eukaryotic RNA (cytosine-5)-methyltransferases. Genomics, 93: 350-357 • Pavlopoulou A, Kossida S (2007) Plant cytosine-5 DNA methyltransferases: structure, function, and molecular evolution. Genomics, 90 :530-541*Corresponding author #Equal contribution |
Collaborations
www.compgen.org/home
dielectricsgroup.physics.ntua.gr/georgakilas
 |
brianicarr@hotmail.com |
OVERVIEW
Hepatocellular carcinoma (HCC) appears to consist of several poorly-defined phenotypes, with several distinct poor prognostic factors, such as high alpha-fetoprotein (AFP) and presence of portal vein thrombosis (PVT). Recently, systemic inflammation has been identified as a major independent poor prognosis factor, based on the Glasgow score that utilizes serum C-reactive protein (CRP) and albumin levels. This implicated the tumor microenvironment (inflammation) in prognosis and thus as an influence on the biology of the tumor itself.
RESEARCH INTERESTS
Our over-all aims are to: A, identify distinct clinical HCC profiles/phenotypes; to B, study the effects of inflammatory mediator influence on HCC growth and invasion in vitro; and C, to continue our previous studies on multikinase drug resistance in HCC cells and to learn how to enhance the effects of these agents on HCC growth inhibition.
RESEARCH HIGHLIGHTS
We have collaborated with a group of 15 Turkish institutions that treat HCC, to assemble a 1700 HCC patient database for phenotype analysis. We have published one paper (in Oncology) with 3 more in press, to show that Turkish HCC, which is predominantly HBV based- and thus similar to Chinese but not Western HCC in this respect- is broadly similar to western HCC, with regard to presence of PVT, importance of AFP and our identification of the characteristics of that 50% of patients that do not have elevated AFP levels. Interestingly, HCC in different regions of Turkey may have marked variations, such as the high incidence of HDV infection in HCC patients in the Diyabakir region.
We have worked on the significance of albumin and C-reactive protein (CRP) on HCC biology. We reasoned that to have such important clinical prognostic significance (high CRP and low albumin predict poor outcomes). These 2 proteins must have effects. Directly or indirectly on HCC biology. In 2017 we published 2 papers to show that low albumin levels were associated with more aggressive tumor parameters of larger tumors and more portal vein invasion, that could explain the poor prognosis 8by contrast, higher serum albumin levels were associated with smaller tumors). We followed this up, by showing that pure albumin in tissue culture could slow HCC cell line growth a limit invasiveness. This sets the scene for future experiments to test these ideas in animals. We have recently shown that high CRP levels are associated with larger and more aggressive tumors in Turkish HCC patients (in press) and we are beginning to study the actions of pure CRP on and in (CRP is synthesized by cells of liver origin) HCC cells in culture.
In collaboration with the labs of Profs Nese Atabey and Esra Erdal, we are working in the development of Regorafenib-resistant and double Regorafenib/Sorafenib resistant HCC cell lines, to examine both the peculiarities of their cell signaling compared to the parental cells and also to identify possible mechanisms of limiting their resistance to drug growth-inhibitory actions. Furthermore, since both Sorafenib and Regorafenib are FDA-approved for treating HCC patients, they are quite toxic (multiple side-effects) and thus we are examining ways to enhance the effects on HCC cells of both drugs when used at low concentrations.
| PUBLICATIONS (from Pubmed) Full list and citations: Google Scholar: Brian Carr • Carr BI, Guerra V, Steel JL, Lin SN. A comparison of patients with hepatitis B- or hepatitis C-based advanced stage hepatocellular carcinoma. Seminars in Oncology 2015; 42: 309-315. • Pancoska P, Skala L, Nesetril J, Carr BI. Evaluation of total HCC lifespan, including both clinically evident and pre-clinical development, using combined Network Phenotyping Strategy and Fisher information analysis. Seminars in Oncology 2015; 42: 339-346 • Carr BI Viruses and Cancer: Guest Editorial Seminars in Oncology 2015; 42: 189-90 • D’Alessandro R, Refolo MG, Lippolis C, Giannuzzi G, Carella N, Messa C, Cavallini A, Carr BI. Modulation of Regorafenib effects on HCC cell lines by epidermal growth factor. Cancer Chemotherapy and Pharmacology 2015; APRIL 24 EPUB • Facciorusso A, Licinio R, Carr BI, Di Leo A, Barone M. MEK 1/2 inhibitors in the treatment of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2015 Apr 27:1-11 • D’Alessandro R, Messa C, Refolo MG, Carr BI. Modulation of sensitivity and resistance to multikinase inhibitors by microenvironmental platelet factors in HCC. Expert Opinion on Pharmacotherapy. 2015; 16: 2773-2780 • Lippolis C, Refolo MG, D’Alessandro R, Carella N, Messa C, Cavallini A, Carr BI. Resistance to multikinase inhibitor actions mediated by insulin like growth factor-1. J Exp Clin Cancer Res. 2015; 34 (1):90. PMID: 26329608 • Pančoška P, Skála L, Nešetřil J, Carr BI. Validation of the Concept of a Common Typical Time of Disease Duration for Hepatocellular Carcinoma Patients Using the Fisher Information Processing of Tumor Imaging Results Combined With Network Phenotyping Strategy Quantification of Individual Patient Clinical Profile Patterns. Semin Oncol. 2015;42:672-8. PMID: 26320070 • Carr BI and Guerra V. Low alpha-fetoprotein levels are associated with improved survival in hepatocellular carcinoma patients with portal vein thrombosis. Dig. Dis. Sciences 2016; 61:937-947 • Carr BI and Guerra V. HCC extrahepatic metastasis in relation to tumor size and ALKP levels. Oncology 2016; 90:136-142 • Carr BI and Guerra V. A Hepatocellular Carcinoma Aggressiveness Index and its relationship to liver enzyme levels. Oncology 2016; 90:215-220 • Modulation of doxorubicin actions in hepatocellular carcinoma cells by Insulin-like Growth Factor-I. Biochemistry & Analytical Biochemistry 2016;5: 256. doi:10.4172/2161- 1009.1000256 • Yilmaz Y, Erdal Y, Atabey N, Carr BI. Platelets, microenvironment and hepatocellular carcinoma: a review. Biochemistry and Analytical Biochemistry 2016;5;2. doi:10.4172/2161-1009.1000281 • Mazzocca A, Ferraro G, Misciagna G, Carr BI. A systemic evolutionary approach to cancer: hepatocarcinogenesis as paradigm. Medical Hypotheses 2016; 93: 132-137 • Carr BI, Guerra V, Giannini EO et al. An HCC Aggressiveness Index and blood GTP, bilirubin and platelet levels. J. Integrative Oncology 2016; 5:172. doi:10.4172/2329-6771.1000172 • Pinato DJ et al. The ALBI grade provides objective hepatic reserve phenotyping across each BCLC stage of hepatocellular carcinoma. J. Hepatology 2017; 66: 338-346 • Rozen R, Menachem Y, Carr BI, Shibolet O. Liver Transplantation for a Patient with Hepatocellular Carcinoma with Vascular Invasion and Exceeding Milan Criteria-Happy End Despite it all. J Gastrointest Cancer. 2016 Nov 11. [Epub ahead of print] • Carr BI, Guerra V, Giannini EG et al A Liver Index and its relationship to indices of HCC Aggressiveness. J. Integrative Oncology 2016; 5:3 DOI: 10.4172/2329-6771.1000178 • Marino IR, Carr BI. New Developments in Orthotopic Liver Transplant for Hepatocellular Carcinoma. Exp Clin Transplant. 2017 Mar;15 (Suppl 2):1-6 • Bağırsakçı E, Sahin E, Atabey N , Erdal E, Carr BI. Role of Albumin in growth inhibition in Hepatocellular Carcinoma. Oncology May 2017; 93: 136-142 • Carr BI, Guerra V. Validation of a Liver Index and its Significance for HCC Aggressiveness. J. Gastrointestinal Cancer 2017; June 20; doi: 10.1007/s12029-017-9971-4. EPub • Carr BI, Guerra V. Serum albumin levels in relation to tumor parameters in hepatocellular carcinoma patients. Int J. Biol Markers 2017; 32: e391-e396 • Akkiz H, Carr BI, Yalcun K, Guerra V et at. Characteristics of hepatocellular carcinoma aggressiveness factors in Turkish patients. Oncology 2017|; Dec 6. doi: 10.1159/000484564. [Epub ahead of print]. PMID: 29207378 • Carr BI, Akkiz, H, Uskudar O, Yalcin K et al. HCC with low- and normal- alphafetoprotein levels. Clinical Practice 2018 (in press) • D’Alessandro R, Refolo MG, Lippolis C, Carella N, Messa C, Cavallini A, Carr BI. Strong enhancement by an IGF1-R antagonist of migration inhibition due to Sorafenib, vitamin K1 or both in hepatocellular carcinoma cell lines. Cellular Oncology 2018 (in press) |
 |
nese.atabey@deu.edu.tr Members Ezgi Bağırsakçı, MSc Student Mesude Angın, MSc Student Dehan Çömez, MSc Student Gülsün Bağcı, MSc Student |
OVERVIEW
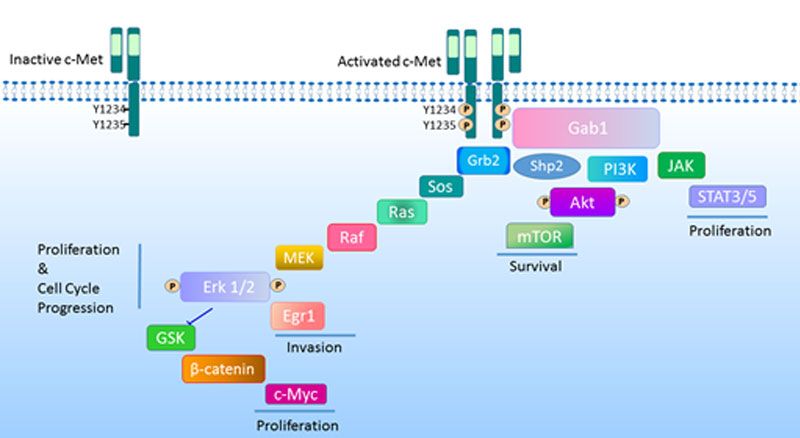
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer and the third leading cause of cancer-related deaths worldwide. Hepatocyte growth factor (HGF)/c-Met signaling is particularly important in the development of HCC as it controls growth, survival, migration and differentiation of tumor cells. Others and we have shown that abnormalities in HGF/c-Met signaling are implicated in tumor development and progression in a variety of cancers. The increase in c-Met expressing stem/progenitor cells in the liver is required for hepatocarcinogenesis, and high c-Met expression is correlated with metastasis, poor prognosis, and drug resistance. An elevated HGF level is uncommon in tumorigenesis except for breast tumors. Therefore, ligand-independent c-Met activation in HCC is a critical phenomenon that should be investigated further.
RESEARCH INTERESTS
Our group is focused on signaling pathways that modulate the growth, motility, and invasion of hepatocellular carcinoma and using this molecular knowledge to improve the diagnosis and treatment of HCC. Our aim is to understand the molecular mechanisms behind the aggressive behavior and drug resistance in HCC and to translate this molecular knowledge to improve diagnosis and treatment.
Currently, our projects encompass:
1) The molecular mechanisms of HGF/c-Met mediated invasion and metastasis in HCC,
2) The effects of tumor microenvironment on the regulation of signaling networks and cellular behaviors of HCC,
3) The roles of non-coding RNAs in the regulation of c-Met signaling in HCC,
4) Fabrication and validation of a new lab-on-a-chip device for early diagnosis of metastasis.
In the next 5 years, we aim to:
• Identify the role of HGF/c-Met signaling in the regulation of glucose metabolism in HCC and further use this knowledge for developing strategies to prevent HCC.
• Understand the functional role of the HGF/c-Met axis in acquired sorafenib resistance in HCC cells and to improve targeted therapies of HCC patients.
• Fabricate and validate a Lab-on-a-chip (LOC) system that can predict the metastatic and invasive ability to circulate tumor cells (CTCs) in HCC and that can also determine the effects of drugs on tumor metastasis.
Lab members:
Atabey lab is currently seeking highly motivated PhD students. If interested, please contact nese.atabey@deu.edu.tr
| PUBLICATIONS (selected) Full list and citations : Google Scholar : Nese Atabey • İşcan E, Güneş A, Korhan P, Yılmaz Y, Erdal E, Atabey N. The regulatory of heparin on c-Met signaling in hepatocellular carcinoma cells. J Cell Commun Signal. 2016 Dec 14. [Epub ahead of print] • Saygideger-Kont Y, Minas TZ, Jones H, Hour S, Celik H, Temel I, et al. Ezrin Enhances EGFR Signaling and Modulates Erlotinib Sensitivity in Non-Small Cell Lung Cancer Cells. Neoplasia. 2016;18(2):111-20. doi: 10.1016/j.neo.2016.01.002. PubMed PMID: 26936397; PubMed Central PMCID: PMCPMC5005263. • Firtina Karagonlar Z, Koc D, Sahin E, Avci ST, Yilmaz M, Atabey N, et al. Effect of adipocyte-secreted factors on EpCAM+/CD133+ hepatic stem cell population. Biochemical and biophysical research communications. 2016;474(3):482-90. doi: 10.1016/j.bbrc.2016.04.137. PubMed PMID: 27131739. • Firtina Karagonlar Z, Koc D, Iscan E, Erdal E, Atabey N. Elevated HGF Expression as an Autocrine c-Met Activation Mechanism in Acquired Resistance to Sorafenib in HCC Cells. Cancer Sci. 2016 Jan 20. doi: 10.1111/cas.12891. • Karagonlar ZF, Korhan P, Atabey N. Targeting c-Met in Cancer by MicroRNAs: Potential Therapeutic Applications in Hepatocellular Carcinoma. Drug Dev Res. 2015 Nov;76(7):357-67. doi: 10.1002/ddr.21274. • Gunes A, Iscan E, Topel H, Avci ST, Gumustekin M, Erdal E, Atabey N. Heparin treatment increases thioredoxin interacting protein expression in hepatocellular carcinoma cells. Int J Biochem Cell Biol. 2015; 65:169-8. • Korhan P, Erdal E, Kandemiş E, Cokaklı M, Nart D, Yılmaz F, Can A, Atabey N. Reciprocal activating crosstalk between c-Met and caveolin 1 promotes invasive phenotype in hepatocellular carcinoma. PLoS One. 2014; 9(8): e105278. • Korhan P, Erdal E, Atabey N. miR-181a-5p is downregulated in hepatocellular carcinoma and suppresses motility, invasion and branching-morphogenesis by directly targeting c-Met. Biochem Biophys Res Commun. 2014; 450 (4):1304-12. • Bozkaya G, Korhan P, Cokakli M, Erdal E, Sagol O, Karademir S, Korch C, Atabey N. Cooperative interaction of MUC1 with the HGF/c-Met pathway during hepatocarcinogenesis. Mol Cancer. 2012;11: 64. • Ozen E, Gozukizil A, Erdal E, Uren A, Bottaro DP, Atabey N. Heparin inhibits Hepatocyte Growth Factor induced motility and invasion of hepatocellular carcinoma cells through early growth response protein 1. PLoS One. 2012;7:e42717. • Kunter I, Erdal E, Nart D, Yilmaz F, Karademir S, Sagol O, Atabey N. Active form of AKT controls cell proliferation and response to apoptosis in hepatocellular carcinoma. Oncol Rep. 2014;31(2):573-80. • Gumustekin M, Kargi A, Bulut G, Gozukizil A, Ulukus C, Oztop I, Atabey N. HGF/c-Met overexpressions, but not met mutation, correlates with progression of non-small cell lung cancer. Pathol Oncol Res. 2012 (2):209-18. |
| PATENTS • Bottaro, D. P., Giubellino A., Atabey S. N., Soriano J. V., Breckenridge D. E.ve Burke T. R. (2011). Inhibition of cell motility, angiogenesis, and metastasis, Pub No: US7871981 B2. • Atabey, S. N., Bottaro D. P., Breckenridge D. E., Gao Y., Soriano J. V.ve Yao Z. J. (2001). Inhibition of cell motility and angiogenesis by inhibitors of the Grb2 SH2-domain, Pub No: WO2001028577 A3. |
 |
Ayşim GÜNEŞ, PhD a.gkizil@hotmail.com |
 |
Erkan KAHRAMAN, PhD erkankahraman@gmail.com |
 |
Hande Topel
|
 |
Yeliz YILMAZ yelizyg@gmail.com |
Etkinlik başvurularınız için aşağıdaki formu doldurduktan sonra lütfen etkinlik sorumlumuza e-posta ile iletiniz.
Cell Culture Room
B008 Sterilization chamber
Use of all devices in laboratory B005
| ANA SAYFA | YÖNERGE | ÇALIŞMA İZNİ ÖN TALEP FORMU | BAŞVURU FORMU | ÜYELER | İLETİŞİM |
| Prof.Dr. Ensari GÜNELİ | Başkan | |
| Prof.Dr. H. Alper BAĞRIYANIK | Başkan Yardımcısı | |
| Prof. Dr. Belgin ÜNAL | Üye | |
| Doç.Dr. Ralph Meuwissen | Üye | |
| Doç.Dr. H. Güneş ÖZHAN | Üye | |
| Yrd.Doç.Dr. Ayşe Banu DEMİR | Üye | |
| Uzm. Umur KELEŞ | Üye | |
| Hakkı Muammer KARAGÖZ | Üye | |
| Uzm. Kerem ESMEN | Üye | |
| Bilg. İşlt. Tuba AKÜNAL | Sekreter |
| HOME | DIRECTIVES | PRE-REQUEST FORM | APPLICATION FORM | MEMBERS | CONTACT |
| Prof. Ensari GÜNELİ | Chair | |
| Prof. H. Alper BAĞRIYANIK | Vice-Chair | |
| Prof. Belgin ÜNAL | Member | |
| Assoc.Prof. Ralph Meuwissen | Member | |
| Assoc.Prof. H. Güneş ÖZHAN | Member | |
| Assist.Prof. Ayşe Banu DEMİR | Member | |
| Spec. Umur KELEŞ | Member | |
| Hakkı Muammer KARAGÖZ | Member | |
| Spec. Kerem ESMEN | Member | |
| Comp.Op. Tuba AKÜNAL | Secretary |
| HOME | DIRECTIVES | PRE-REQUEST FORM | APPLICATION FORM | MEMBERS | CONTACT |
Click here to see İBG_AELEC 2017 Meeting Calendar.
[vfb id=21]
| HOME | DIRECTIVES | PRE-REQUEST FORM | APPLICATION FORM | MEMBERS | CONTACT |
 |
DOKUZ EYLUL UNIVERSITY IZMIR INTERNATIONAL BIOMEDICINE AND GENOME INSTITUTE DIRECTIVES OF THE LOCAL ETHIC COMITTEE FOR ANIMAL EXPERIMENTS (IBG-AELEC) |
 |
|
OBJEVTIVES The aim of this protocol is to determine the acceptable minimal ethical standards, to deliver opinion, demarcate the borders for applications, to assess the applications from this point of view for keeping, raising, making observations on them, producing, using for experiments, isolating from nature and transporting of all animals that are used for experiments, experimental research, training, testing and health service applications at DEU, iBG or other institutions. By forming a Laboratory Animals Local Ethics Committee (HAYDEK) it aims to give judgment about ethics principles; and to inspect, approve and (where necessary) to terminate research proposals, including the essential procedures, and the presentation of planned studies. EXTENT (1) The protocol contains, all required permissions to be taken before the application to DEU, iBG and other institutions where animals are used for experimental purposes, establishment of Dokuz Eylul University Local Animal Ethics Committee for this reason, The procedures and principles of the work, the tasks of the establishment and the training, supervision and obligations. (2) (1) This protocol does not contain:
BASIS This protocol is established with regards to the;
DESCRIPTIONS As it is in this directive;
FORMATION OF THE LOCAL ETHICS COMMITTEE FOR ANIMAL EXPERIMENTS (IBG-AELEC), QUALIFICATIONS AND ASSİGNMENT OF THE MEMBERS (1) FORMATION OF THE LOCAL ETHICS COMMITTEE FOR ANIMAL EXPERIMENTS (IBG-AELEC), QUALIFICATIONS AND ASSİGNMENT OF THE MEMBERS The IBG-AELEC is composed of:
(2) ASSIGNMENT TO IBG-AELEC and TERM OF OFFICE
IBG-AELEC WORKING METHOD
DUTIES of IBG’s HADYEK
AUTHORISATION AND OPERATION PRINCIPLES of IBG-AELEC IBG-AELEC works in line with the following principles;
PRACTICES RELATED TO ANIMAL EXPERIMENTS AND RESEARCH LABORATORY ANIMALS (1) Issues regarding the animals used in the researh applied to IBG-AELEC:
SEVERITY CLASSIFICATION OF THE EXPERIMENTS, ANESTHESIA AND IMPLEMENTATION OF ANESTHESIA, ACT OF KILLING AND OTHER RELATED HANDLINGS DURING The procedures related to anesthesia and its implementation, killing and severity classification of experiments are carried out in accordance with the articles 21 and 22, and Annex-8 and Annex-9 of the Directive on Protection And Welfare Of Laboratory Animals Used For Experiments and Other Scientific Purposes published in the Official Gazette dated 13/12/2011 and numbered 28141 by Ministry of Food, Agriculture and Livestock. RE-USE OF ANIMALS IN THE EXPERIMENTS (1) Reuse of an animal previously used in one or more experiments is allowed in the following situations:
(2) In exceptional cases, after the examination of the animal by a veterinarian and by excluding subparagraph (a), an animal may be allowed to be re-used in an experiment involving severe pain, suffering or equivalent discomfort provided that the animal will not be used more than once. TERMINATION OF EXPERIMENTS (1) Every experiment is terminated when no more observations on the experiment can be performed, or when genetically modified animal breed or species are no longer be traced and If pain, suffering, and permanent damage equal to or more than the one needle penetration on an ongoing basis is expected, the experiments are terminated. (2) At the end of an experiment, a veterinarian decides if an animal should survive. If an animal continues to survive, appropriate care and accommodation services are provided for its health condition. If the animal continues to live with moderate or severe pain, suffering, distress, and permanent damage, its life is terminated. PROJECT APPLICATIONS Projects to be accepted to IBG-AELEC:
The Applicantions to the Ethical Committee is done by filling out the Turkish or English version of the “Experimental Animal Ethics Committee Application Form” and submitting it to IBG-AELEC secretariat. The applications for thesis studies are made by the academic advisor as the coordinator of the project and in the other research projects, applications are done by project coordinators. The applications to be made from outside the university are done with the official letter of the project manager from his institution. RESPONSIBILITIES OF THE APPLICANT In the application to the Committee, personal declaration is essential and the applicants sign a commitment form.
PROPERTIES AND PROJECT FORMAT IN PROJECT APPLICATION (1) The research project should describe the purpose, design, methodology, statistical aspects and organization of the work to be done. The projects are edited in accordance with scientific criteria so as not to exceed 4-6 pages. The name of the research, type (experimental, preliminary study, thesis study, etc.) the name of the researchers, their place of employment, the contact address should be on the first page. (2) Project Format Filling out the application form: Title page: The project name, the institutions of participating researchers, tasks and signatures, the research, and the contact details, department and etc.of the project coordinator. Project Title: must be clear, short, and scientific that describes the whole project without abbreviations or jargons. Project Summary: It should include goals of the project, the objectives, the materials/methods, the predicted damages and benefits, the species and the trait of the animals to be used in accordance with the 3R principles. Introduction: The project’s rationale with relevant literature should be given in detail. Literature should be placed in the text according to the order of use. Objective: The objective of the project reflecting the basic hypothesis should be written clearly and briefly. Method: The research design, the experimental groups and the justifications for the study design, the drug doses, the route of administration, the place where the drugs are obtained, the literature on drugs if available, the amount of biological samples to be taken, the sampling and storage method, details on evaluation and test methods to be performed with literary support if available, selecting appropriate statistical method, surgical procedure to be performed on animals, brief information on postoperative care, methods of evaluation of laboratory findings, detailed technical information should be given on the place where the animals are to be kept, the conditions of the animals being kept and the permission certificate from the laboratory where testing is carried out, the method of termination of the experiment, time, cages and the companies where the necessary materials and equipment are obtained should be given in details. Innovations that the project will bring: The innovations and contributions that the project will bring to the literature should be stated briefly. References: Must be written in the order of use in the project and must be written according to the Index Medicus format at the end of the project. Project coordinator should submit the application to IBG-AELEC by adding his or her letter (Annex 1). The first application should include:
EVALUATION OF PROJECTS AND DECISION MAKING (1) Projects are evaluated according to following criteria:
(2) The people to do the project evaluation for IBG-AELEC should be chosen regarding their competence in experimental design, animal experimentation or animal care and nutrition. (3) The project evaluation must be transparant. The project evaluation is carried out in an impartial manner for the protection of intellectual property rights and confidential information. (4) A member of IBG-AELEC is appointed as a reporter for each appropriate application. The reporter evaluates the project and explains his/her assessment to the Committee members at the meeting. The Board discusses the project. If the committee deems necessary, it can invite the researcher in charge to the meeting and ask questions and points that need explanation. (5) After the project evaluation, IBG-AELEC makes its decision as ‘approved’, ‘demands revision’, ‘conditional approval’ and ‘not approved’. If one of the Ethics Committee Members participates in the project, he/she should not vote on that project. The applications that receive ‘demands revision’ is re-evaluated by the Ethics Committee after the applicant revises the project. The projects that receive ‘conditional approval’ will be monitored for a specific time that is determined by the decision of the Ethics Committee. Then the fulfillment of the required conditions will be re-evaluated and the decision will be finalized whether the project is ‘approved or ‘not approved. IBG-AELEC may evaluate whether the persons who will be practicing in laboratory animals are competent or not and if a person is believed not to have enough experience IBG-AELEC may offer the applicant to work with an experienced researcher in the field for a specific time to gain experience. (6) The Ethics Committee approvals are given for 5 years and are valid for this period. Additional time may be requested for the projects that cannot be completed within this period. (7) In the research protocol, it is mandatory to notify the ethics committee and obtain its approval to make changes after the initial Ethics Committee approval. No changes in a protocol can be made without the approval of the ethics committee. The study will be stopped if the protocol changes that are not approved by the ethics committee are implemented. (8) All researchers working in the units of Dokuz Eylul University who will do research in the fields related to the activities described in Article 1 of “Dokuz Eylul University-Animal Experiments Local Ethics Committee Directive” are required to obtain the approval of IBG-AELEC with a written application. (9) All national and international scientific publications related to the activities described in the Article 1 of IBG-AELEC Directive, are required to include the statement “Approved by the Ethics Committee” or “Complies with the Ethics Committee Principles”. (10) The responsibility of the ethical issues originating from declaring “Approved by the Ethics Committee” or “Complies with the Ethics Committee Principles”, which appears in all national and international scientific publications of research related to the activities described in the Article 1 of IBG-AELEC Directive, without applying the Ethics Committee, belongs to the authors(s) of the publication. RETROSPECTIVE ASSESSMENT (1) The following aspects can be evaluated retrospectively in the documents reported to IBG-AELEC:
(2) All projects where non-human primates are used, including procedures with long-lasting and untreatable severe pain, suffering, and agony,, torture and misery, are assessed retrospectively. (3) Projects other than the provisions of paragraph 2 can be exempted from retrospective assessment. TRAINING OF THE PERSONNEL DEALING WITH LABORATORY ANIMALS In accordance with the Article 7/c of this directive, IBG-AELEC determines whether the persons who will deal with laboratory animals are qualified or not and then issues “Certificate in Laboratory Animal Science” to the persons who are trained according to the regulations executed by the Ministry of Forestry and Water Management. of Laboratory animal users can not perform experiments, training or to test procedures on these animals or keep these animals in their work places. In case the person who plans to use laboratory animals in the work submitted for approval has no certificate, the work is not approved. The persons who apply to get a “Certificate in Laboratory Animal Science” are required to fulfill the following conditions:
LAST TERMS The correspondences of the ethics committee are confidential. No information is provided to third parties except for the authorized institutions described by the related legislation and the Directive of TR Ministry of Forestry and Water Affairs on Local Animal Ethics Committee Work Plan and Essentials. ENFORCEMENT This Directive is presented to the Central Ethical Committee for Animal Experiments after being approved by the Decision of the Senate of Dokuz Eylül University and shall enter into force on the date of its approval by the Central Ethical Committee for Animal Experiments. EXECUTION The terms of this directory are executed by the Rector of DEU. |
||
| HOME | DIRECTIVES | PRE-REQUEST FORM | APPLICATION FORM | MEMBERS | CONTACT |
IBG-AELEC Secretariat
Tuba AKUNAL
tuba.akunal@deu.edu.tr
+90(232) 412 67 70
www.ibg.deu.edu.tr/en/ibg-aelec
Address
Izmir International Biomedicine and Genome Institute (iBG-izmir)
Dokuz Eylul Universitesi Saglik Yerleskesi
Balcova 35340 Izmir/TURKEY
| HOME | DIRECTIVES | PRE-REQUEST FORM | APPLICATION FORM | MEMBERS | CONTACT |
The researchers who will apply for an Ethics Committee Approval, have to submit their forms to the Ethics Committee Secretariat after uploading their projects to the system.
Click here to see İBG_AELEC 2017 Meeting Calendar.
Please fill in the Application Form, “Conflict of Interest” and “IBG-AELEC contract” after reading the section below.
Application Requirements:
The application documents are as follows:
1- Approved Experimental Study Permit Pre-Request Form
2- The Application Form, the Contract at the end of the form will be signed by all researchers.
3- “Conflict of Interest” is to be signed by all researchers
4- Resume and Certificate in Laboratory Animal Science of one of the researchers
5- Minimum 3 maximum 7 literature related to the subject.
You can find the application documents below:
| Application Form | Contract | Conflict of Interest | Letter of Application |
Please click here to upload the documents you have filled.
| HOME | DIRECTIVES | PRE-REQUEST FORM | APPLICATION FORM | MEMBERS | CONTACT |
Researchers who will apply for Ethics Committee Approval are required to obtain “Experimental Study Permit” approval before completing the Ethics Committee Application form. Please download the “Experimental Study Permit Pre-Request Form” from the link below.
| Experimental Study Permit Pre-Request Form |
Click here to see İBG_AELEC 2017 Meeting Calendar.
You can upload the completed form to the system here.
[vfb id=’22’]
| HOME | DIRECTIVES | PRE-REQUEST FORM | APPLICATION FORM | MEMBERS | CONTACT |
IBG-HADYEK has been established to conduct ethical review on research proposals for all experimental, testing and educational purposes involving animal experimentation in iBG-izmir and all processes related to production, maintenance, housing and transport of experimental animals.
IBG-HADYEK application process is as follows:
Dokuz Eylul University Izmir International Biomedicine and Genome Institute is established on 09/03/2015 by the Council of Ministers after the approval of the University Senate.
The Institute has two departments: Genome Sciences and Molecular Biotechnology and Biomedicine and Health Technologies. Genome Sciences and Molecular Biotechnology has M.Sc., Ph.D. and Integrated Ph.D. programs in “Molecular Biology and Genetics”.
Our programs are interdisciplinary and international. The institute provides students with the opportunity to conduct basic and translational research.
All of the programs are taught in English.
Dokuz Eylül Üniversitesi İzmir Uluslararası Biyotıp ve Genom Enstitüsü üniversite Senatosu tarafından onaylanarak, 09/03/2015 tarihinde Bakanlar Kurulu Kararı ile kurulmuştur.
Enstitünün Genom Bilimleri ve Moleküler Biyoteknoloji ve Biyotıp ve Sağlık Teknolojileri isimli iki anabilim dalı mevcuttur. Genom Bilimleri ve Moleküler Biyoteknoloji A.D.’da “Moleküler Biyoloji ve Genetik” Yüksek Lisans, Doktora ve Bütünleşik Doktora programları bulunmaktadır.
Programlarımız disiplinler arası ve uluslararası özellik taşımaktadır. Enstitümüzde öğrencilere temel ve uygulamalı araştırmaların ön planda olduğu araştırmalar yapma olanağı sunulmaktadır.
Programların tamamında öğretim dili İngilizcedir.
| ANA SAYFA | YÖNERGE | ÇALIŞMA İZNİ ÖN TALEP FORMU | BAŞVURU FORMU | ÜYELER | İLETİŞİM |
Etik Kurul Onayı almak için başvuracak araştırıcıların Etik Kurul Başvuru formunu doldurmadan önce “Deneysel Çalışma İzni” onayı almaları gerekmektedir. Lütfen aşağıdaki bağlantıdan “Deneysel Çalışma İzni Ön Talep Formu”nu indirerek dikkatlice doldurunuz.
| Deneysel Çalışma İzni Ön Talep Formu |
İBG-HADYEK 2017 Toplantı Takvimini görmek için tıklayın.
Doldurmuş olduğunuz ön talep formunu aşağıdan sisteme yükleyebilirsiniz.
[vfb id=’22’]
| ANA SAYFA | YÖNERGE | ÇALIŞMA İZNİ ÖN TALEP FORMU | BAŞVURU FORMU | ÜYELER | İLETİŞİM |
İBG-HADYEK 2017 Toplantı Takvimini görmek için tıklayın.
[vfb id=21]
| ANA SAYFA | YÖNERGE | ÇALIŞMA İZNİ ÖN TALEP FORMU | BAŞVURU FORMU | ÜYELER | İLETİŞİM |
Hadyek Sekreterya
www.ibg.deu.edu.tr/tr/ibg-hadyek
Adres
İzmir Uluslararası Biyotıp ve Genom Enstitüsü (iBG-izmir)
Dokuz Eylül Üniversitesi Sağlık Yerleşkesi
Balçova 35340 İzmir
| ANA SAYFA | YÖNERGE | ÇALIŞMA İZNİ ÖN TALEP FORMU | BAŞVURU FORMU | ÜYELER | İLETİŞİM |
 |
DOKUZ EYLÜL ÜNİVERSİTESİ İZMİR ULUSLARARASI BİYOTIP VE GENOM ENSTİTÜSÜ HAYVAN DENEYLERİ YEREL ETİK KURULU YÖNERGESİ |
 |
|
AMAÇ Bu Yönergenin amacı, İzmir Uluslararası Biyotıp ve Genom Enstitüsü (İBG), Dokuz Eylül Üniversitesinde (DEÜ) ve müracaat durumunda diğer kurum ve kuruluşlarda bilimsel deneysel araştırma, test, sağlık hizmetleri uygulamaları, eğitim-öğretim amaçlı olarak kullanılacak tüm deney hayvanlarının barındırılmaları, üretilmeleri, doğadan izole edilmeleri, nakledilmeleri, deneylerde kullanılmaları ve üzerlerinde gözlem yapılması ile ilgili tüm süreçlerde etik bakımdan kabul edilebilir minimum standartları saptamak, etik ilkeler doğrultusunda görüş bildirmek, uygulamaların sınırlarını belirlemek ve araştırma önerilerini bu açıdan incelemek üzere oluşturulacak “Deney Hayvanları Yerel Etik Kurulu”nun (HADYEK) kuruluş ve çalışma esaslarına, yapılması planlanan işlemlerin sunulmasına, araştırma önerilerinin incelenmesine, uygulanmalarına izin verilmesine, uygulamaların izlenmesine ve gerektiğinde sonlandırılmasına ilişkin esasları belirlemektir. KAPSAM (1) Yönerge İBG, DEÜ ve başvuru durumunda diğer kurum ve kuruluşlarda deney amacıyla kullanılacak hayvanların kullanımından önce alınması gereken izinleri, bu amaçla DEÜ İBG-HADYEK’in oluşturulmasını, çalışma usul ve esaslarını, kurulun görevleri ile eğitim, denetim ve yükümlülüklerini kapsar. (2) Bu Yönerge:
DAYANAK Bu Yönerge;
TANIMLAR Bu Yönerge’de geçen;
İBG-HADYEK’in OLUŞUMU, ÜYELERİN NİTELİKLERİ ve ATANMASI (1) İBG-HADYEK OLUŞUMU ve ÜYELERİN NİTELİKLERİ
(2) İBG-HADYEK’e ATANMA ve GÖREV SÜRELERİ
İBG-HADYEK’in ÇALIŞMA ŞEKLİ
İBG-HADYEK’in GÖREVLERİ
İBG-HADYEK’in YETKİLERİ VE ÇALIŞMA İLKELERİ İBG-HADYEK aşağıda belirtilen ilkeler doğrultusunda çalışır;
DENEY HAYVANLARI VE ARAŞTIRMALARA İLİŞKİN UYGULAMALAR (1) İBG-HADYEK’e başvuran çalışmalarında kullanılacak hayvanlara ilişkin hususlar:
ANESTEZİ VE ANESTEZİ UYGULANMASI, ÖLDÜRME VE DENEYLERDE ŞİDDET SINIFLANDIRMASI İLE İLGİLİ İŞLEMLER Anestezi ve anestezi uygulanması, öldürme ve deneylerde şiddet sınıflandırması ile ilgili işlemler Gıda, Tarım ve Hayvancılık Bakanlığınca 13/12/2011 tarihli ve 28141 sayılı Resmî Gazete’de yayımlanan Deneysel ve Diğer Bilimsel Amaçlar İçin Kullanılan Hayvanların Refah ve Korunmasına Dair Yönetmeliğin 21 inci ve 22 nci maddeleri ile Ek-8 ve Ek-9’a göre yapılır. HAYVANLARIN DENEYLERDE TEKRAR KULLANIMI (1) Daha önce bir ya da birkaç deneyde kullanılan bir hayvanın tekrar kullanılmasına aşağıdaki durumlarda izin verilir:
(2) İstisnai durumlarda, (a) bendini uygulama dışı bırakacak şekilde ve hayvanın veteriner hekim tarafından muayene edilmesinden sonra, hayvanın şiddetli acı, ızdırap veya eşdeğerini içeren bir deneyde birden fazla kullanılmaması şartıyla bir hayvanın tekrar kullanılmasına izin verilebilir. DENEYİN SONLANDIRILMASI (1) Deneyle ilgili olarak daha fazla gözlemin yapılamayacağı hallerde ya da genetiği değiştirilmiş hayvan soyları ve nesilleri artık takip edilmiyorsa veya sürekli devam eden bir şekilde iğne batırılmasına eşdeğer ya da daha fazla acı, eziyet, ızdırap ve kalıcı hasar yaşaması bekleniyorsa deney sonlandırılır. (2) Deneyin sonunda, bir hayvanın yaşamaya devam etmesine dair karar bir veteriner hekim tarafından alınır. Bir hayvanın yaşatılmaya devam etmesi durumunda, sağlık durumuna uygun bakım ve barınma hizmeti sağlanır. Hayvan orta veya şiddetli acı, eziyet, ızdırap ve kalıcı hasar ile yaşamaya devam ediyorsa yaşamına son verilir. PROJELERİN BAŞVURULARI İBG-HADYEK’e kabul edilecek projeler:
Kurula başvurular, Türkçe ya da İngilizce “Deney Hayvanları Etik Kurulu Başvuru Formu” doldurularak İBG-HADYEK sekreterliğine yapılır. Başvuruların, tez çalışmalarında yürütücü sıfatıyla danışman öğretim üyesi, diğer araştırma projelerinde ise proje yürütücüsü tarafından yapılır. Üniversite dışından yapılacak başvurular ise proje yürütücüsünün kendi kurumundan alacağı resmi üst yazı ile yapılır. BAŞVURU SAHİPLERİNİN SORUMLULUKLARI Kurula başvurularda kişisel beyan esastır, başvuranlar taahhüt formu imzalar.
PROJE BAŞVURUSUNDA ARANACAK ÖZELLİKLER ve PROJE FORMATI (1)Araştırma projesi, yapılacak çalışmanın amacını, tasarımını, yöntemini, istatistiksel yönleri ve örgütlenmesini tanımlamalıdır. Projeler bilimsel kriterlere uygun olarak 4-6 sayfayı geçmeyecek şekilde hazırlanır. Araştırma adı, türü (deneysel, ön çalışma, tez çalışması vb) araştırmacıların adı, görev yerleri, iletişim adresi ilk sayfada olmalıdır. (2)Proje formatı Başlık sayfası: Proje ismi / Katılan araştırmacıların kurumu, görevi ve imzaları/ Araştırmanın Türü ve yürütücünün iletişim bilgileri/Anabilim Dalı vb. Proje Başlığı: Projenin tamamını tanımlayan kısaltma ve jargonlardan uzak, kısa, açık ve bilimsel olamalıdır. Proje Özeti: Amaç, materyal-metot, tahmin edilen hasar ve faydalar ile kullanılan hayvanların tür ve soy özellikleri, projenin hedefleri, 3R ilkesine uygun olarak yazılmalıdır. Giriş: Projenin yapılma gerekçesi ve ilgili literatür bilgileri ayrıntılı bir şekilde verilmelidir. Literatürler kullanım sırasına göre metin içinde yerleştirilmelidir. Amaç: Projenin temel hipotezini yansıtan amacı net ve kısaca yazılmalıdır Yöntem: Araştırma dizaynı, deney grupları ve oluşturulma gerekçesi, ilaç dozları, ilaçların veriliş yolu, ilaçların nereden temin edildiği, varsa literatürü, alınacak biyolojik örneklerin miktarı, örnekleme ve saklama şekli, yapılacak değerlendirme ve test yöntemlerinin literatür desteği ile ayrıntılı bir şekilde verilmesi, uygun istatistik yöntemin seçilmesi, hayvanlar üzerinde yapılacak cerrahi prosedür, post-operatif bakım, laboratuar bulguların değerlendirme yöntemlerinin kısaca açıklanması, hayvan temin edilen yer, hayvanların barındırılma şartları ve deney yapılacak laboratuardan izin belgesi, deney sonlandırma yöntemi, zamanı, kafesleme, gerekli malzeme ve gereçlerin elde edildiği firmalar gibi detaylı teknik bilgi verilmelidir. Projenin getireceği yenilikler: Projenin literatüre getireceği yenilikler ve katkılar kısaca belirtilmelidir. Kaynaklar: Kullanım sırasına göre metne yerleştirilerek ve proje sonunda Index Medicus formatına göre yazılmalıdır. Hazırlanan projeler; proje yürütücüsünün dilekçesi ile İBG-HADYEK’e başvurulur. İlk başvuruda bulunması gerekenler:
PROJELERİN DEĞERLENDİRİLMESİ ve KARAR VERİLMESİ (1) Projeler aşağıda belirtilen kriterlere göre değerlendirilir.
(2) İBG-HADYEK tarafından proje değerlendirmesini yapacak kişilerin; 3R ilkesi, deney tasarımı, hayvan deneyleri veya hayvan bakım ve beslenmesi konusunda yetkin olmasına göre seçilmesine dikkat edilir. (3) Proje değerlendirmesi şeffaf olmalıdır. Fikri mülkiyet haklarının ve gizli bilgilerin korunması için, proje değerlendirmesi tarafsız bir şekilde gerçekleştirilir. (4) Uygun başvurular için İBG-HADYEK üyelerinden biri raportör olarak atanır. Raportör projeyi inceler ve toplantıda kurul üyelerine değerlendirmesini açıklar. Kurulda proje tartışılır. Kurul gerekli görürse, çalışmayı yapmak isteyen araştırmacıyı toplantıya çağırarak soruları ve açıklama gereken noktaları sorabilir. (5) İBG-HADYEK, yaptığı değerlendirme sonucunda proje hakkında yapılması uygun, düzeltilmesi gerekir, koşullu olarak uygun, ya da yapılması uygun değildir şeklinde karar verir. Etik Kurul üyelerinin isimleri olan projelerde ilgili kurul üyesi oy kullanamaz. Düzeltilmesi gerekir kararı verilen başvurular, başvuru sahibi tarafından düzeldikten sonra, Etik Kurulda tekrar değerlendirilir. Koşullu olarak uygun kararı verilen projeler Etik Kurul tarafından belirlenecek belli bir süre boyunca izlenip, istenen koşulların yerine getirilip getirilmediği değerlendirildikten sonra uygun ya da uygun değildir şeklinde karara bağlanır. İBG-HADYEK, deney hayvanlarında uygulama yapacak kişilerin ehil olup olmadıklarını da değerlendirebilir ve yeterli görmemesi halinde eğitim almaları için alanlarında deneyimli bir araştırıcıyla/uzmanla bir süre çalışmalarını isteyebilir. (6) Etik Kurul onayları 5 yıl için verilir. Bu süre içinde onaylar geçerlidir. Bu süre içinde bitirilemeyen projeler için ek süre talep edilebilir. (7) Araştırma protokolünde, Etik Kurul onayından sonra yapılmak istenen değişikliklerin Etik Kurula yazılı olarak bildirilmesi ve onayının alınması zorunludur. Etik kurulun onayı alınmaksızın hiçbir protokol değişikliği uygulanamaz. Çalışmada etik kurulca onaylanmamış protokol değişikliklerinin uygulanması durumunda çalışma durdurulur. (8) Dokuz Eylül Üniversitesi birimlerinde, Dokuz Eylül Üniversitesi İBG-HADYEK Yönergesi Madde 1’de yer alan etkinliklerle ilgili uygulama yapacak olan tüm araştırmacılar yazılı olarak başvurarak İBG-HADYEK onayı almak zorundadırlar. (9) İBG-HADYEK Yönergesi Madde-1’de yer alan etkinliklerle ilgili araştırmalara ilişkin ulusal ya da uluslararası nitelikli her türlü bilimsel yayında “Etik Kurul Onayı Alınmıştır” veya “Etik Kurul İlkelerine Uyulmuştur” ifadesine yer verilmek zorundadır. (10) Etik Kurula başvurulmadan İBG-HADYEK Yönergesi Madde- 1’de yer alan etkinliklerle ilgili araştırmalara ilişkin ulusal ya da uluslararası nitelikli her türlü bilimsel yayında yer alacak olan “Etik Kurul Onayı Alınmıştır” veya “Etik Kurul İlkelerine Uyulmuştur” beyanından doğabilecek etik sorunların tüm sorumluluğu, yayının yazar / yazarlarına aittir. GERİYE DÖNÜK DEĞERLENDİRME (1) İBG-HADYEK’e bildirilen dokümanlara göre geriye dönük aşağıdaki hususlar değerlendirilir:
(2) İnsan dışı primatların kullanıldığı tüm projeler ve uzun süreli ve iyileştirilemeyen şiddetli ağrı, eziyet ve ızdırap içeren prosedürler de dahil “şiddetli” olarak sınıflandırılan prosedürleri içeren projeler geriye dönük değerlendirmeye tabi tutulur. (3) İkinci fıkra hükümleri dışındaki projeler geriye dönük değerlendirmeden muaf tutulabilir. DENEY HAYVANI İLE UĞRAŞACAK PERSONELİN EĞİTİMİ İBG-HADYEK, bu Yönergenin 7/c maddesi gereği deney hayvanı ile çalışılacak kişilerin ehil olup olmadıklarını belirler, T.C. Orman ve Su İşleri Bakanlığınca düzenlenen mevzuata uygun olarak ‘Deney Hayvanı Kullanım Sertifikası’ verir. Deney hayvanı kullanıcıları, sertifika almadan bu hayvanlar üzerinde deney, eğitim, test amacıyla işlem yapamaz ve çalışma mekanlarında bu hayvanları barındıramazlar. Kurulun onayına sunulan çalışmalarda deney hayvanı kullanan kişinin kullanım sertifikası bulunmaması halinde kurulca çalışmaya onay verilmez. “Deney Hayvanı Kullanım Sertifikası” almak için kurula başvuranların aşağıdaki şartları taşımaları gereklidir.
SON HÜKÜMLER Etik Kurul yazışmaları gizli olup, ilgili mevzuat ve Hayvan Deneyleri Etik Kurullarının Çalışma Usul ve Esaslarına Dair Yönetmelik’te belirtilen yetkili kurumlar dışında üçüncü şahıslara bilgi verilemez. YÜRÜRLÜK Bu Yönerge, Dokuz Eylül Üniversitesi Senatosu Kararı ile kabul edildikten sonra Hayvan Deneyleri Merkezi Etik Kurulu’na sunulur ve bu Kurul tarafından onaylandığı tarihten itibaren yürürlüğe girer. YÜRÜTME Bu Yönerge hükümleri DEÜ Rektörü tarafından yürütülür. |
||
| ANA SAYFA | YÖNERGE | ÇALIŞMA İZNİ ÖN TALEP FORMU | BAŞVURU FORMU | ÜYELER | İLETİŞİM |
Etik Kurul Onayı almak için başvuracak araştırıcıların projelerini, sisteme yükledikten sonra iBG-izmir Hayvan Deneyleri Etik Kurulu Sekreterliği’ne teslim etmeleri gerekmektedir.
İBG-HADYEK 2017 Toplantı Takvimini görmek için tıklayın.
Lütfen aşağıdaki başvuru koşulları bölümünü okuduktan sonra Başvuru Formunu ve Taahhütnameyi doldurunuz.
Başvuru Koşulları
Kurulumuza müracaat edenlerin hazırlayacakları Başvuru Belgeleri şunlardır:
1- Onaylanmış Deneysel Çalışma İzni Ön Talep Formu
2- Başvuru Formu, Form sonundaki taahhütname tüm araştırıcılar tarafından imzalanacak.
3- Çıkar İlişkisi Belgesi (tüm araştırıcılar tarafından imzalanacak)
4- Araştırıcılardan birinin özgeçmişi ve Deney Hayvanı Kullanım Sertifikası
5- Konuyla ilgili en az 3 en fazla 7 tane literatür.
Başvuru dökümanlarına aşağıdan ulaşabilirsiniz.
| Başvuru Formu | Taahhütname | Çıkar ilişkisi belgesi | Başvuru Dilekçesi |
Doldurmuş olduğunuz dökümanları sisteme yüklemek için lütfen tıklayınız.
| ANA SAYFA | YÖNERGE | ÇALIŞMA İZNİ ÖN TALEP FORMU | BAŞVURU FORMU | ÜYELER | İLETİŞİM |
iBG-HADYEK, iBG-izmir’de gerçekleştirilecek hayvan deneyi içerikli tüm deneysel, test ve eğitim amaçlı araştırmalar ve deney hayvanlarının üretim, bakım, barındırma ve nakledilme ile ilgili tüm süreçlerin etik olarak gözden geçirilmesi ve araştırma önerilerini incelemek üzere kurulmuştur.
iBG-HADYEK başvuru süreci aşağıdaki gibidir.
| Core Manager |
|
iBG-Izmir biopharmaceuticals unit has the caliber to provide service to the biotech industries for complete upstream and downstream process development including cell line development. We have perfect team of scientist with industrial and academic back ground with the capability to deliver and execute to meet the defined product quality.
We provide the following services below for biotech industries. If you are looking for the recombinant protein or biosimilar product development and/or for small scale manufacturing for preclinical studies please contact us we will be happy to help you.

Brian I. Carr, Hikmet Akkiz, Oguz Uskudar, et al. HCC with low- and normal serum alpha-fetoprotein levels. Clinical Practice (Therapy) 2018; 15(1): 453-464.
Serhat Tozburun, Cedric Blatter, Meena Siddiqui, Eelco F. J. Meijer, and Benjamin J. Vakoc. Phase-stable Doppler OCT at 19 MHz using a stretched-pulse mode-locked laser. Biomedical Optics Express 2018; 9(3): 952-961. doi: 10.1364/BOE.9.000952.
Meena Siddiqui, Ahhyun S. Nam, Serhat Tozburun*, Norman Lippok, Cedric Blatter & Benjamin J. Vakoc. High-speed optical coherence tomography by circular interferometric ranging. Nature Photonics 2018 Jan 26; 12: 111–116. doi: 10.1038/s41566-017-0088-x.
Brian I. Carr, Hikmet Akkiz, Oguz Üsküdar et al. HCC with low- and normal serum alpha-fetoprotein levels. Clinical Practice (Therapy) 2018; 15(1): 453-464. doi: 10.4172/clinical-practice.1000393.
Sag D, Özkan M, Kronenberg M, Wingender G. Improved Detection of Cytokines Produced by Invariant NKT Cells. Sci Rep 2017 Nov 30; 7(1):16607. doi: 10.1038/s41598-017-16832-1.
U. Blache, J. Guerrero, S. Güven, A.S. Klar, A. Scherberich. Microvascular Networks and Models, In vitro Formation, in: W. Holnthoner, A. Banfi, J. Kirkpatrick, H. Redl (Eds.),. Vascularization for Tissue Engineering and Regenerative Medicine. Springer International Publishing Cham, 2017, pp. 1-40.
Cigdem Ozen, Meltem Ceylan Unlusoy, Nazanin Aliary, Mehmet Ozturk, Oya Bozdag Dundar. Thiazolidinedione or Rhodanine: A Study on Synthesis and Anticancer Activity Comparison of Novel Thiazole Derivatives. J Pharm Pharm Sci 2017 Nov; 20 (1): 415-427. doi:10.18433/J38P9R.
Gökhan Karakülah. RTFAdb: A database of computationally predicted associations between retrotransposons and transcription factors in the human and mouse genomes. Genomics 2017 Nov; 17. doi:10.1016/j.ygeno.2017.11.002.
Carr BI, Guerra V. Serum albumin levels in relation to tumor parameters in hepatocellular carcinoma patients. Int J Biol Markers 2017 Oct 31;32(4):e391-e396. doi:10.5301/ijbm.5000300.
Refolo MG, D’Alessandro R, Lippolis C, Carella N, Cavallini A, Messa C, Carr BI. IGF-1R tyrosine kinase inhibitors and Vitamin K1 enhance the antitumor effects of Regorafenib in HCC cell lines. Oncotarget 2017 Sep 30; 8(61):103465-103476. doi:10.18632/oncotarget.21403.
Onur Tokel, Ahmet Turnalı, Ghaith Makey, et al. In-chip microstructures and photonic devices fabricated by nonlinear laser lithography deep inside silicon. Nature Photonics 2017 Sep; 11: 639–645. doi:10.1038/s41566-017-0004-4.
Ozen C, Ceylan-Unlusoy M, Ozturk M, Bozdag-Dundar O. A novel chromonyl thiohydantoin with anti-proliferative action on primary hepatocellular carcinoma cells. Med Chem Res 2017 Sep; 1-8. doi:10.1007/s00044-017-2037-0.
Yuqing Zhu, Vahid Serpooshan, Sean Wu, Utkan Demirci, Pu Chen, Sinan Güven. Tissue Engineering of 3D Organotypic Microtissues by Acoustic Assembly. In: Methods in Molecular Biology. Humana Press 2017 Sep; 1-12. doi: 10.1007/7651_2017_68.
Lina Zelinger, Gökhan Karakülah*, Vijender Chaitankar, Jung-Woong Kim, Hyun-Jin Yang, Matthew J. Brooks, Anand Swaroop. Regulation of Noncoding Transcriptome in Developing Photoreceptors by Rod Differentiation Factor NRL. Investigative Ophthalmology & Visual Science 2017 Sep; 58: 4422-4435. doi: 10.1167/iovs.17-21805.
Ozturk M, Batur T, Ekin U, Erdogan A, İscan E, Keles U, Oz O, Ozen C. Molecular Pathogenesis of Liver Cancer. J Gastrointest Cancer. 2017 Jul 17 (Review). doi: 10.1007/s12029-017-9957-2. [Epub ahead of print].
Carr BI, Guerra V. Serum albumin levels in relation to tumor parameters in hepatocellular carcinoma patients. Int J Biol Markers 2017 Aug 28. doi: 10.5301/ijbm.5000300.
Ors A, Papin C, Favier B, Roulland Y, Dalkara D, Ozturk M, Hamiche A, Dimitrov S, Padmanabhan K. Histone H3.3 regulates mitotic progression in mouse embryonic fibroblasts. Biochem Cell Biol 2017 Aug; 95(4):491-499. doi: 10.1139/bcb-2016-0190.
Yılmaz Y, Güneş A, Topel H, Atabey N. Signaling Pathways as Potential Therapeutic Targets in Hepatocarcinogenesis. J Gastrointest Cancer 2017 Aug 18. doi: 10.1007/s12029-017-9958-1.
Carr BI, Guerra V. Validation of a Liver Index and Its Significance for HCC Aggressiveness. J Gastrointest Cancer 2017 Jun 20. doi: 10.1007/s12029-017-9971-4.
Ezgi Karaca*, João P.G.L.M. Rodrigues, Andrea Graziadei, Alexandre M.J.J. Bonvin, Teresa Carlomagno. M3: an integrative framework for structure determination of molecular machines. Nature Methods 2017. doi: 10.1038 / nmeth.4392.
Alural B, Ayyildiz ZO, Tufekci KU, Genc S, Genc K. Erythropoietin Promotes Glioblastoma via miR-451 Suppression. Vitam Horm. 2017;105:249-271. doi: 10.1016/bs.vh.2017.03.002. Epub 2017 Apr 19. PubMed PMID: 28629521.
Gökhan Karakülah. Discovery and Annotation of Plant Endogenous Target Mimicry Sequences from Public Transcriptome Libraries: A Case Study of Prunus persica. Journal of Integrative Bioinformatics. 2017 (in press), doi: 10.1515/jib-2017-0009.
Sezgin E, Azbazdar Y, Ng XW, Teh C, Simons K, Weidinger G, Wohland T, Eggeling C, Ozhan G. Binding of canonical Wnt ligands to their receptor complexes occurs in ordered plasma membrane environments. FEBS J. 2017 Aug; 284(15): 2513-2526. doi: 10.1111/febs.14139.
Akyerli C., Yuksel S., Can O., Erson-Omay Z., Oktay Y.*, Cosgun E., Ulgen E., Erdemgil Y., San A., von Deimling A., Gunel M., Yakicier C., Pamir M.N., Ozduman K. Use of telomerase promoter mutations to mark specific molecular subsets with reciprocal clinical behavior in IDH mutant and IDH wild-type diffuse gliomas. Journal of Neurosurgery. 2017 Jun; 16:1-13. doi: 10.3171/2016.11.
Schneider F, Waithe D, Clausen MP, Galiani S, Koller T, Ozhan G, Eggeling C, Sezgin E. Diffusion of lipids and GPI-anchored proteins in actin-free plasma membrane vesicles measured by STED-FCS. Mol Biol Cell. 2017 Jun 1;28(11): 1507-1518. doi: 10.1091/mbc.E16-07-0536.
Oktay Y., Boylu C.A., Özduman K. Gliom Gelişiminde Genetik Yatkınlığın Rolü. Türk Nöroşirürji Dergisi. 2017 May; 27(2): 122-130.
Gökhan Karakülah, Aslı Suner. PlanTEnrichment: A tool for enrichment analysis of transposable elements in plants. Genomics. 2017 (in press), doi:10.1016/j.ygeno.2017.05.008.
Bağırsakçı E., Şahin E., Atabey N., Erdal E. & Carr B.I. Role of Albumin in growth inhibition in Hepatocellular Carcinoma. Oncology. 2017 May 10. doi:10.1159/000471807.
Gümürdü A., Yildiz R., Eren E., Karakülah G., Ünver T., Genç Ş. & Park Y. MicroRNA exocytosis by large dense-core vesicle fusion. Scientific Reports. 2017 Mar 30; 7:45661. doi:10.1038/srep45661.
Marino IR, Carr B.I. New Developments in Orthotopic Liver Transplant for Hepatocellular Carcinoma. Exp Clin Transplant. 2017 Mar;15(Suppl 2):1-6.
Alural B, Genc S, Haggarty S. Diagnostic and therapeutic potential of microRNAs in neuropsychiatric disorders: Past, present, and future. Prog Neuropsychopharmacol Biology Psychiatry. 2017 Feb; 6;73:87-103 doi: 10.1016/j.pnpbp.2016.03.010. Epub 2016 Apr 9. PubMed PMID: 27072377; PubMed Central PMCID: PMC5292013.
Pinato D., Sharma R., Allara E., Yen C., Arizumi T., Kubota K., Bettinger D., Jang J.W., Smirne C., Kim Y.W., Kudo M., Howell J., Ramaswami R., Burlone M.E., Guerra V., Thimme R., Ishizuka M., Stebbing J., Pirisi M. & Carr B.I. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J. Hepatology, 2017 Feb; 66(2): 338-346. doi:http://dx.doi.org/10.1016/j.jhep.2016.09.008.
van Waalwijk van Doorn LJ, Gispert JD, Kuiperij HB, et al. Improved Cerebrospinal Fluid-Based Discrimination between Alzheimer’s Disease Patients and Controls after Correction for Ventricular Volumes. J Alzheimers Dis. 2017 Jan 24; 56(2):543-555. doi: 10.3233/JAD-160668
Park CG, Park Y & Suh BC. The HOOK region of voltage-gated Ca2+ channel β subunits senses and transmits PIP2 signals to the gate. J Gen Physiol. 2017 Jan 13. doi:10.1085/jgp.201611677.
Arslan N, Guzel O, Kose E, Yılmaz U, Kuyum P, Aksoy B, Çalık T. Is ketogenic diet treatment hepatotoxic for children with intractable epilepsy? Seizure. 2016 Dec; 43: 32-38. doi:10.1016/j.seizure.2016.10.024.
Ebru Diler, Turgay Unver & Gökhan Karakülah. Differential Expression of Hyperhydricity Responsive Peach miRNAs. Journal of Integrative Bioinformatics. 2016 Dec; 13(5): 308. doi:10.2390/biecoll-jib-2016-308.
İşcan E, Güneş A, Korhan P, Yılmaz Y, Erdal E, Atabey N. The regulatory role of heparin on c-Met signaling in hepatocellular carcinoma cells. J Cell Commun Signal. 2016 Dec 14; 1-12. doi:10.1007/s12079-016-0368-0.
Gökhan Karakülah, Kuaybe Yücebilgili Kurtoğlu, Turgay Unver. PeTMbase: A Database of Plant Endogenous Target Mimics (eTMs). PLoS ONE. 2016 Dec 9; 11(12): e0167698. doi:10.1371/journal.pone.0167698.
Calibasi Kocal G, Güven S, Foygel K, Goldman A, Chen P, Sengupta S, Paulmurugan R, Baskin Y, Demirci U. Dynamic Microenvironment Induces Phenotypic Plasticity of Esophageal Cancer Cells Under Flow. Sci Rep. 2016 Dec 2; 6:38221. doi: 10.1038/srep38221.
Jung-Woong Kim, Hyun-Jin Yang, Matthew John Brooks, Lina Zelinger, Gökhan Karakülah*, Norimoto Gotoh, Alexis Boleda, Linn Gieser, Felipe Giuste, Dustin Thad Whitaker, Ashley Walton, Rafael Villasmil, Jennifer Joanna Barb, Peter Jonathan Munson, Koray Dogan Kaya, Vijender Chaitankar, Tiziana Cogliati, Anand Swaroop. NRL-Regulated Transcriptome Dynamics of Developing Rod Photoreceptors. Cell Reports. 2016 Nov 22; 17(9): 2460-2473. doi:http://dx.doi.org/10.1016/j.celrep.2016.10.074.
Arslan N, Kose E, Guzel O. The Effect of Ketogenic Diet on Serum Selenium Levels in Patients with Intractable Epilepsy. Biol Trace Elem Res. 2016 Nov 21. doi:10.1007/s12011-016-0897-7.
Erbayraktar Z, Alural B, Erbayraktar RS, Erkan EP. Cell division cycle 7-kinase inhibitor PHA-767491 hydrochloride suppresses glioblastoma growth and invasiveness. Cancer Cell Int. 2016 Nov 18;16:88. eCollection 2016. doi:10.1186/s12935-016-0364-8.
Muhammad Ayaz Mustufa, Cigdem Ozen, Imran Ali Hashmi, Afshan Aslam, Jameel Ahmed Baig, Gokhan Yildiz, Shoaib Muhammad, Imam Bakhsh Solangi, Naim ul Hasan Naqvi, Mehmet Ozturk and Firdous Imran Ali. Synthesis and bio-molecular study of (+)-N-Acetyl-α-amino acid dehydroabietylamine derivative for the selective therapy of hepatocellular carcinoma. BMC Cancer. 2016, Nov 14; 16: 883. doi:10.1186/s12885-016-2942-5.
Büyüköz M, Erdal E, Alsoy Altinkaya S. Nanofibrous gelatin scaffolds integrated with NGF-loaded alginate microspheres for brain tissue engineering. J Tissue Eng Regen Med. 2016 Nov 12. doi: 10.1002/term.2353.
Jeannot V, Busser B, Vanwonterghem L, Michallet S, Ferroudj S, Cokol M, Coll JL, Ozturk M, Hurbin A. Synergistic activity of vorinostat combined with gefitinib but not with sorafenib in mutant KRAS human non-small cell lung cancers and hepatocarcinoma. OncoTargets and Terapy. 2016, Nov 9; 9: 6843—6855. doi:10.2147/OTT.S117743.
Carr B.I., Guerra V., Giannini E.G. et al. A Liver Index and its Relationship to Indices of HCC Aggressiveness. J Integr Oncol. 2016 Oct;5(4). pii: 178. doi: 10.4172/2329-6771.1000178.
Erkan D, Kayali HA. Replacement of Soybean Meal with Animal Origin Protein Meals Improved Ramoplanin A2 Production by Actinoplanes sp. ATCC 33076. Appl Biochem Biotechnol. 2016 Sep;180(2):306-21. doi:10.1007/s12010-016-2100-1.
Hani Alotaibi, Nese Atabey, Kasım Diril, Esra Erdal, Mehmet Ozturk. Molecular Mechanisms of Hepatocellular Carcinoma, Chapter Hepatocellular Carcinoma Part of the series Current Clinical Oncology. Springer. 2016, Aug 27; 43-63. doi:10.1007/978-3-319-34214-6_3.
Alagoz Y., Gurkok T., Zhang B. & Unver T. Manipulating the Biosynthesis of Bioactive Compound Alkaloids for Next-Generation Metabolic Engineering in Opium Poppy Using CRISPR-Cas 9 Genome Editing Technology. Sci Rep. 2016 Aug 3; 6:30910. doi: 10.1038/srep30910.
Bakır Y., Eldem V., Zararsız G. & Unver T. Global Transcriptome Analysis Reveals Differences in Gene Expression Patterns Between Nonhyperhydric and Hyperhydric Peach Leaves. Plant Genome. 2016 Jul; 9(2). doi:10.3835/plantgenome2015.09.0080.
Marsano A, Medeiros da Cunha CM, Ghanaati S, Gueven S, Centola M, Tsaryk R, Barbeck M, Stuedle C, Barbero A, Helmrich U, Schaeren S, Kirkpatrick JC, Banfi A, Martin I. Spontaneous In Vivo Chondrogenesis of Bone Marrow-Derived Mesenchymal Progenitor Cells by Blocking Vascular Endothelial Growth Factor Signaling. Stem Cells Transl Med. 2016 Jul 26. doi: 10.5966/sctm.2015-0321.
Pavlopoulou A., Oktay Y., Vougas K., Louka M., Vorgias C.E., Georgakilas A.G. Determinants of resistance to chemotherapy and ionizing radiation in breast cancer stem cells. Cancer Letters, 2016, Jul 19; 380(2):485-493. doi: 10.1016/j.canlet.2016.07.018.
Carr B.I., Guerra V., Giannini E.O. et al. An HCC Aggressiveness Index and Blood GTP, Blirubin and Platelet Levels. J. Integrative Oncology. 2016 Jun 20; 5:172. doi: 10.4172/2329-6771.1000172.
Oktay Y., Ülgen E., Can Ö., Akyerli C.B., Yüksel Ş., Erdemgil Y., Durası I.M., Henegariu O.I., Nanni E.P., Selevsek N., Grossmann J., Erson-Omay E.Z., Bai H., Gupta M., Lee W., Turcan Ş., Özpınar A., Huse J.T., Sav M.A., Flanagan A., Günel M., Sezerman O.U., Yakıcıer M.C., Pamir M.N. & Özduman K. IDH-mutant glioma specific association of rs55705857 located at 8q24.21 involves MYC deregulation. Sci Rep. 2016 Jun 10; 6: 27569. doi: 10.1038/srep27569.
Yilmaz Y, Atabey N, Erdal E & Brian I. Carr. Platelets, Microenvironment and Hepatocellular Carcinoma. Biochemistry & Analytical Biochemistry, 2016 June 29; 5:281 (Review). doi:10.4172/2161-1009.1000281.
Antonio Mazzoccaa, Giovanni Ferrarob, Giovanni Misciagnac, Brian I. Carr. A systemic evolutionary approach to cancer: Hepatocarcinogenesis as a paradigm. Medical Hypotheses, 2016, Aug; 93: 132-137. doi:10.1016/j.mehy.2016.05.027.
Karakülah G, Karakuş M, Suner A, Demir S, Arserim SK, Töz S, Özbel Y. sandflyDST: a dynamic web-based decision support tool for the morphological identification of sandflies present in Anatolia and mainland Europe, and user study. Medical and Veterinary Entomology, 2016 June 24. doi:10.1111/mve.12182.
Can Küçük, Xiaozhou Hu, Qiang Gong, Bei Jiang, Adam Cornish, Philippe Gaulard, Timothy McKeithan, Wing C. Chan. Diagnostic and Biological Significance of KIR Expression Profile Determined by RNA-Seq in Natural Killer/T-Cell Lymphoma. The American Journal of Pathology, 2016 June; 186(6): 1435-1441. doi:10.1016/j.ajpath.2016.02.011.
Zeynep Firtina Karagonlar, Doğukan Koç, Eren Şahin, Sanem Tercan Avci, Mustafa Yilmaz, Neşe Atabey, Esra Erdal. Effect of adipocyte-secreted factors on EpCAM+/CD133+ hepatic stem cell population. Biochemical and Biophysical Research Communications, 2016 June 3; 474(3): 482-490. doi:10.1016/j.bbrc.2016.04.137.
Yakup Bakır, Vahap Eldem, Gökmen Zararsız, Turgay Unver. Global Transcriptome Analysis Reveals Significant Differences in Gene Expression Patterns Between The Non-Hyperhydric and Hyperhydric Leaves of Prunus persica. The Plant Genome, 2016, May; 9(2): 1-9.
Salimi R, Yener N, Safari R. Use and Evaluation of Newly Synthesized Fluorescence Probes to Detect Generated OH• Radicals in Fibroblast Cells. J Fluoresc. 2016 May; 26(3):919-24. doi: 10.1007/s10895-016-1780-9.
Firtina Karagonlar Z, Koc D, Iscan E, Erdal E, Atabey N. Elevated hepatocyte growth factor expression as an autocrine c‐Met activation mechanism in acquired resistance to sorafenib in hepatocellular carcinoma cells. Cancer Sci. 2016 Apr; 107(4):407-416 doi: 10.1111/cas.12891.
Carr BI & Guerra V. A Hepatocellular Carcinoma Aggressiveness Index and Its Relationship to Liver Enzyme Levels. Oncology 2016 Mar 15; 90(4). doi: 10.1159/000444394.
Gao LM, Zhao S, Liu WP, Zhang WY, Li GD, Küçük C, Hu XZ, Chan WC, Tang Y, Ding WS, Yan JQ, Yao WQ, Wang JC. Clinicopathologic Characterization of Aggressive Natural Killer Cell Leukemia Involving Different Tissue Sites. Am J Surg Pathol, 2016 Mar 11. doi: 10.1097/PAS.0000000000000634.
Carr BI & Guerra V. Low Alpha-Fetoprotein Levels Are Associated with Improved Survival in Hepatocellular Carcinoma Patients with Portal Vein Thrombosis. Digestive Diseases and Sciences, 2016 Feb 26; 61(3):937-947. doi: 10.1007/s10620-015-3922-3.
Ferroudj S, Yildiz G, Bouras M, Iscan E, Ekin U, Ozturk M. Role of fanconi anemia/BRCA pathway genes in hepatocellular carcinoma chemoresistance. Hepatol Res. 2016 Feb 16. doi: 10.1111/hepr.12675.
Carr BI & Guerra V. Hepatocellular Carcinoma Extrahepatic Metastasis in Relation to Tumor size and Alkaline Phosphatase Levels. Oncology 2016 Feb 12; 90(3):1-7. doi: 10.1159/000443480.
Durmaz I, Guven EB, Ersahin T, Ozturk M, Calis I, Cetin-Atalay R. Liver cancer cells are sensitive to Lanatoside C induced cell death independent of their PTEN status. Phytomedicine. 2016 Jan 15; 23(1):42-51. doi: 10.1016/j.phymed.2015.11.012.
Wingender G. From the deep sea to everywhere? Environmental antigens for iNKT cells. Arch Immunol Ther Exp, 2015 Dec 24 (Review). doi: 10.1007/s00005-015-0381-7.
Güzel O, Yılmaz U, Uysal U, Arslan N. The effect of olive oil-based ketogenic diet on serum lipid levels in epileptic children. Neurol Sci. 2015 Dec 23; 1-6. doi: 10.1007/s10072-015-2436-2.
Namkoong B, Guven S, Ramesan S, Liaudanskaya V, Abzhanov A, Demirci U. Recapitulating cranial osteogenesis with neural crest cells in 3-D microenvironments. Acta Biomaterialia, 2015 Dec 07. doi: 10.1016/j.actbio.2015.12.004.
Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, Herrley E, Rasquinha N, McArdle S, Wu R, Peluso E, Metzger D, Ichinose H, Shaked I, Chodaczek G, Biswas SK, Hedrick CC. Patrolling Monocytes Control Tumor Metastasis to the Lung. Science. 2015, Nov 20; 350(6263): 985-90.
Wingender G & Kronenberg M. Characterization of human T cell subsets via surface markers. Cytometry A, 2015, Oct 27; 87A; 1067-1069.
D’Alessandro R, Messa C, Refolo MG, Carr BI. Modulation of sensitivity and resistance to multikinase inhibitors by microenvironmental platelet factors in HCC. Expert Opinion on Pharmacotherapy. 2015 Oct 19; 16(18):1-8. doi: 10.1517/14656566.2015.1101065.
Wingender G, Sag D, Kronenberg M. NKT10 cells: a novel iNKT cell subset. Oncotarget. 2015 Sept 29; 6(29): 26552-26553 (Review). doi: 10.18632/oncotarget.5270.
Wingender G, Birkholz A, Sag D, Farber E, Chitale S, Howell AR, Kronenberg M. Selective conditions are required for the induction of iNKT cell hypo-responsiveness by antigenic stimulation. The Journal of Immunology. 2015 Sept 9; 195: 3838-3848. doi: 10.4049/jimmunol.1500203.
Lippolis C, Refolo MG, D’Alessandro R, Carella N, Messa C, Cavallini A, Carr BI. Resistance to multikinase inhibitor actions mediated by insulin like growth factor-1. Journal of Experimental & Clinical Cancer Research. 2015 Sept 2; 34(1):90. doi: 10.1186/s13046-015-0210-1.
Pančoška P, Skála L, Nešetřil J, Carr BI. Validation of the Concept of a Common Typical Time of Disease Duration for Hepatocellular Carcinoma Patients Using the Fisher Information Processing of Tumor Imaging Results Combined With Network Phenotyping Strategy Quantification of Individual Patient Clinical Profile Patterns. Seminars in Oncology. 2015 Aug; 42(4):672-678. doi: 10.1053/j.seminoncol.2015.05.004.
Gunes A, Iscan E, Topel H, Avci ST, Gumustekin M, Erdal E, Atabey N. Heparin treatment increases thioredoxin interacting protein expression in hepatocellular carcinoma cells. The International Journal of Biochemistry & Cell Biology. 2015 Aug; 65:169-181. doi: 10.1016/j.biocel.2015.05.025.
Hu X, Chan WC, Kücük C. Generation of a genetically engineered aggressive nk-cell leukemia cell line with stable IL2 expression. Acta Medica International. 2015 Jul; 2(2):78-84. doi: 10.5530/ami.2015.3.6.
Ozhan G and Weidinger G. Wnt/β-catenin signaling in heart regeneration. Cell Regen (Lond). 2015 Jul 8; 4(1):3. doi: 10.1186/s13619-015-0017-8
Alotaibi H, Basilicata MF, Shehwana H, Kosowan T, Schreck I, Braeutigam C, Konu O, Brabletz T, Stemmler MP. Enhancer cooperativity as a novel mechanism underlying the transcriptional regulation of E-cadherin during mesenchymal to epithelial transition. Biochimica et Biophysica Acta (BBA) – Gene Regulatory Mechanisms. 2015 June; 1849(6):731-742. doi: 10.1016/j.bbagrm.2015.01.005.
Alural B, Ozerdem A, Allmer J, Genc K, Genc S. Lithium protects against paraquat neurotoxicity by NRF2 activation and miR-34a inhibition in SH-SY5Y cells. Front Cell Neurosci. 2015 May 28; 9:209. doi: 10.3389/fncel.2015.00209.
Dilek Cevik, Gokhan Yıldız & Mehmet Ozturk. Common telomerase reverse transcriptase promoter mutations in hepatocellular carcinomas from different geographical locations. World J Gastroenterol. 2015 Jan 7; 21(1): 311–317. doi: 10.3748/wjg.v21.i1.311.
Alural B, Duran GA, Tufekci KU, Allmer J, Onkal Z, Tunali D, Genc K, Genc S. EPO Mediates Neurotrophic, Neuroprotective, Anti-Oxidant, and Anti-Apoptotic Effects via Downregulation of miR-451 and miR-885-5p in SH-SY5Y Neuron-Like Cells. Frontiers in Immunology. 2014 Sep 30; 5:475. doi: 10.3389/fimmu.2014.00475.
* Current address IBG.
TBMM’de 16/02/2015 yılında kabul edilen 9 Mart 2015 tarih ve 29290 sayılı T.C. Resmi Gazete’ de yayımlanan 2015/7321 karar sayısıyla, Dokuz Eylül Üniversitesi Rektörlüğü bünyesinde yer alan Hemodiyaliz-Transplantasyon Enstitüsünün adının, İzmir Uluslararası Biyotıp ve Genom Enstitüsü olarak değiştirilmesi kararlaştırılarak, enstitümüz eğitim-öğretim faaliyetlerine başlamıştır. Enstitümüze ilk öğrenci alımı 2016-2017 eğitim öğretim yılında yapılmıştır.
Enstitümüz bünyesinde Genom Bilimleri ve Biyoteknoloji Anabilimdalı kurulmuş olup eğitim faaliyetlerine başlamıştır. Yüksek lisans ve Doktora öğrencileri bu anabilim dalında eğitim görmektedir. Anabilimdalı bünyesinde görevli enstitüsü kadrosunda bulunan 1 doçent, 3 doktor öğretim üyesi bulunmaktadır. Bu öğretim üyelerimiz akademik faaliyetlerimizi üniversitemizin diğer akademik birimlerinden görevlendirme yapılarak eğitim öğretim faaliyetlerimizi yürütmektedir. Enstitümüz kuruluşunda açılan Biyotıp ve Sağlık Teknolojileri anabilimdalımız eğitim faaliyetlerine henüz başlamamıştır.
16/02/2015 yılında kurulan İzmir Uluslararası Biyotıp ve Genom Enstitüsünde Genom Bilimleri ve Moleküler Biyoteknoloji Anabilim Dalına bağlı Moleküler Biyoloji ve Genetik Yüksek Lisans ve Doktora Programları ile öğretime başlamıştır.
07/04/2015 tarihli 439 toplantı sayı numaralı senato kararı ve 28/04/2015 tarihli YÖK Kurul kararı ile “Genom Bilimleri ve Moleküler Biyoteknoloji Anabilim Dalı” ile “Biyotıp ve Sağlık Teknolojileri Anabilim Dalı” kurulmuştur. Enstititümüz ilk kuruluş aşamasında İzmir Biyotıp ve Genom Merkezi bünyesinde Şubat 2018 tarihine kadar faaliyet göstermiş bu tarihten sonra merkez ve enstitü faaliyetlerini ayrı olarak devam etmektedir.
Enstitümüz kuruluş aşamasındaki üniversite rektörümüz sayın Prof. Dr. Mehmet Füzün, kurucu müdürümüz sayın Prof.Dr. Mehmet Öztürk, Bilim Sanayi ve Teknoloji Bakanı Fikri Işık, İzmir Valisi Mustafa Toprak, Nobel ödülü sahibi Sir Tim Hunt’ın yanı sıra çok sayıda yerli ve yabancı bilim insanının katılımıyla açılmıştır.
Enstititümüz ilk kuruluş aşamasında İzmir Biyotıp ve Genom Merkeziyle birlikte aynı bina içerisinde faaliyetlerine başlamıştır ve şuan faaliyetlerini aynı binada devam ettirmektedir.


Professor Brian Carr was born in Glasgow, Scotland in 1944 and grew up in London.
He graduated medical school at the University of London in 1967 and worked for several years in medicine and then in medical oncology. He realized that medical oncology was confusing and mainly witchcraft and decided to learn how to do research and was apprenticed at the Imperial Cancer Research Labs, London with Renato Dulbecco. After his molecular biology PhD (5′-mRNA capped ends) he moved after his advisor’s Nobel Prize with him to the USA.
He then repeated a solid tumor clinical Fellowship and simultaneous Post-Doc in chemical hepatocarcinogenesis at the McArdle Labs, Madison, WI. His first staff job was for 10 yr at the City of Hope, LA, first as Assistant and then as Associate Professor of Medicine, continuing to work on rodent hepatocarcinogenesis, with special interest in natural growth inhibitors (TGF beta).
He was then invited to set up the first liver cancer (HCC) group within the Liver Transplant Institute in Pittsburgh and was appointed as full Professor with tenure and worked there for 20 yr, both running the clinical HCC service and his lab working on HCC growth regulation.
His clinical interests have been for a long time in developing new HCC therapies, focussing initially on hepatic chemoembolization and subsequently on Yttrium-90 bead hepatic internal radioembolization and more recently on vitamin K as HCC therapy. Currently, he is analyzing HCC databases involving several thousand patients, to identify HCC phenotypes with differing clinical parameter patterns and prognoses. The main findings are that context is key, and that any given parameter (such as serum bilirubin or tumor mass) can only be understood in its total clinical context.
Scientifically, he spent several yr working on K vitamins, since their aberrant use is a key biochemical characteristic of HCC and helps identify one of the most important HCC tumor markers, DCP or des-gamma-carboxy prothrombin. DCP positive and AFP positive tumor cells have quite different regulation, just as AFP positive and AFP negative HCCs have differing phenotypes and prognosis. Vitamin K1 mediates inhibitory phosphorylation of Raf via PKC. Sorafenib is an FDA-approved HCC multikinase inhibitor working through direct Raf inhibition. Thus, vitamin K enhances Sorafenib-mediated Raf inhibition and thus enhances the HCC cell growth inhibition.
The clinical observations of HCC context, have led to the recent observations that blood platelet levels identify phenotypically different HCC patient subsets. This has been developed further by recently examining the actions of platelet lysates as promoters of HCC growth and invasion. This has been shown to be due in large part to platelet content of epidermal growth factor (EGF) and insulin-like growth factor 1 (IGF-1). These factors also modulate HCC growth sensitivity and resistance to multikinase inhibitors. Therefore, the net effect of these therapies depends in part on the tumor (HCC) microenvironment. Thus, clinical observations led to experimental mechanistic observations, that in turn have led to clinical application: translational work.
He has published 296 papers-mainly on HCC and 4 books, 3 of which are on HCC and one on Psychological Aspects of Cancer and has written the chapter on Liver Tumors for the last 3 editions of: Harrison’s Principles of Internal Medicine (19th edition, April 2015).
Contact Information: brianicarr@hotmail.com
The institute started its educational activities after the name of the Hemodialysis-Transplantation Institute founded in accordance with the decision published in the Official Gazette with the number of 2015/7321 on 09/03/2015 is changed into İzmir International Biomedicine and Genome Institute. The first student admission was made in the 2016-2017 academic year.
Genome Sciences and Biotechnology Department was established within the body of our institute and started its educational activities. Graduate and Ph.D. students are educated in this department. There are one associate professor and 3 assistant professors in the department. Biomedicine and Health Technologies department has not yet started training activities yet.
Founded on 16/02/2015, Izmir International Biomedicine and Genome Institute has begun Molecular Biology and Genetics Master and Doctoral Programs in the Department of Genome Sciences and Molecular Biotechnology.
Our institute has been active within İzmir Biomedicine and Genome Center until February 2018 during the first phase of its establishment.
The institute is opened with the participation of many domestic and foreign scientists such as Prof.Mehmet Füzün, the founding rector, our founding director, Prof.Dr. Mehmet Öztürk, Science Industry and Technology Minister Fikri Işık, Izmir Governor Mustafa Toprak as well as Nobel Laureate Sir Tim Hunt.


Brian I. Carr, Hikmet Akkiz, Oguz Uskudar, et al. HCC with low- and normal serum alpha-fetoprotein levels. Clinical Practice (Therapy) 2018; 15(1): 453-464.
Serhat Tozburun, Cedric Blatter, Meena Siddiqui, Eelco F. J. Meijer, and Benjamin J. Vakoc. Phase-stable Doppler OCT at 19 MHz using a stretched-pulse mode-locked laser. Biomedical Optics Express 2018; 9(3): 952-961. doi: 10.1364/BOE.9.000952.
Meena Siddiqui, Ahhyun S. Nam, Serhat Tozburun*, Norman Lippok, Cedric Blatter & Benjamin J. Vakoc. High-speed optical coherence tomography by circular interferometric ranging. Nature Photonics 2018 Jan 26; 12: 111–116. doi: 10.1038/s41566-017-0088-x.
Brian I. Carr, Hikmet Akkiz, Oguz Üsküdar et al. HCC with low- and normal serum alpha-fetoprotein levels. Clinical Practice (Therapy) 2018; 15(1): 453-464. doi: 10.4172/clinical-practice.1000393.
Sag D, Özkan M, Kronenberg M, Wingender G. Improved Detection of Cytokines Produced by Invariant NKT Cells. Sci Rep 2017 Nov 30; 7(1):16607. doi: 10.1038/s41598-017-16832-1.
U. Blache, J. Guerrero, S. Güven, A.S. Klar, A. Scherberich. Microvascular Networks and Models, In vitro Formation, in: W. Holnthoner, A. Banfi, J. Kirkpatrick, H. Redl (Eds.),. Vascularization for Tissue Engineering and Regenerative Medicine. Springer International Publishing Cham, 2017, pp. 1-40.
Cigdem Ozen, Meltem Ceylan Unlusoy, Nazanin Aliary, Mehmet Ozturk, Oya Bozdag Dundar. Thiazolidinedione or Rhodanine: A Study on Synthesis and Anticancer Activity Comparison of Novel Thiazole Derivatives. J Pharm Pharm Sci 2017 Nov; 20 (1): 415-427. doi:10.18433/J38P9R.
Gökhan Karakülah. RTFAdb: A database of computationally predicted associations between retrotransposons and transcription factors in the human and mouse genomes. Genomics 2017 Nov; 17. doi:10.1016/j.ygeno.2017.11.002.
Carr BI, Guerra V. Serum albumin levels in relation to tumor parameters in hepatocellular carcinoma patients. Int J Biol Markers 2017 Oct 31;32(4):e391-e396. doi:10.5301/ijbm.5000300.
Refolo MG, D’Alessandro R, Lippolis C, Carella N, Cavallini A, Messa C, Carr BI. IGF-1R tyrosine kinase inhibitors and Vitamin K1 enhance the antitumor effects of Regorafenib in HCC cell lines. Oncotarget 2017 Sep 30; 8(61):103465-103476. doi:10.18632/oncotarget.21403.
Onur Tokel, Ahmet Turnalı, Ghaith Makey, et al. In-chip microstructures and photonic devices fabricated by nonlinear laser lithography deep inside silicon. Nature Photonics 2017 Sep; 11: 639–645. doi:10.1038/s41566-017-0004-4.
Ozen C, Ceylan-Unlusoy M, Ozturk M, Bozdag-Dundar O. A novel chromonyl thiohydantoin with anti-proliferative action on primary hepatocellular carcinoma cells. Med Chem Res 2017 Sep; 1-8. doi:10.1007/s00044-017-2037-0.
Yuqing Zhu, Vahid Serpooshan, Sean Wu, Utkan Demirci, Pu Chen, Sinan Güven. Tissue Engineering of 3D Organotypic Microtissues by Acoustic Assembly. In: Methods in Molecular Biology. Humana Press 2017 Sep; 1-12. doi: 10.1007/7651_2017_68.
Lina Zelinger, Gökhan Karakülah*, Vijender Chaitankar, Jung-Woong Kim, Hyun-Jin Yang, Matthew J. Brooks, Anand Swaroop. Regulation of Noncoding Transcriptome in Developing Photoreceptors by Rod Differentiation Factor NRL. Investigative Ophthalmology & Visual Science 2017 Sep; 58: 4422-4435. doi: 10.1167/iovs.17-21805.
Ozturk M, Batur T, Ekin U, Erdogan A, İscan E, Keles U, Oz O, Ozen C. Molecular Pathogenesis of Liver Cancer. J Gastrointest Cancer. 2017 Jul 17 (Review). doi: 10.1007/s12029-017-9957-2. [Epub ahead of print].
Carr BI, Guerra V. Serum albumin levels in relation to tumor parameters in hepatocellular carcinoma patients. Int J Biol Markers 2017 Aug 28. doi: 10.5301/ijbm.5000300.
Ors A, Papin C, Favier B, Roulland Y, Dalkara D, Ozturk M, Hamiche A, Dimitrov S, Padmanabhan K. Histone H3.3 regulates mitotic progression in mouse embryonic fibroblasts. Biochem Cell Biol 2017 Aug; 95(4):491-499. doi: 10.1139/bcb-2016-0190.
Yılmaz Y, Güneş A, Topel H, Atabey N. Signaling Pathways as Potential Therapeutic Targets in Hepatocarcinogenesis. J Gastrointest Cancer 2017 Aug 18. doi: 10.1007/s12029-017-9958-1.
Carr BI, Guerra V. Validation of a Liver Index and Its Significance for HCC Aggressiveness. J Gastrointest Cancer 2017 Jun 20. doi: 10.1007/s12029-017-9971-4.
Ezgi Karaca*, João P.G.L.M. Rodrigues, Andrea Graziadei, Alexandre M.J.J. Bonvin, Teresa Carlomagno. M3: an integrative framework for structure determination of molecular machines. Nature Methods 2017. doi: 10.1038 / nmeth.4392.
Alural B, Ayyildiz ZO, Tufekci KU, Genc S, Genc K. Erythropoietin Promotes Glioblastoma via miR-451 Suppression. Vitam Horm. 2017;105:249-271. doi: 10.1016/bs.vh.2017.03.002. Epub 2017 Apr 19. PubMed PMID: 28629521.
Gökhan Karakülah. Discovery and Annotation of Plant Endogenous Target Mimicry Sequences from Public Transcriptome Libraries: A Case Study of Prunus persica. Journal of Integrative Bioinformatics. 2017 (in press), doi: 10.1515/jib-2017-0009.
Sezgin E, Azbazdar Y, Ng XW, Teh C, Simons K, Weidinger G, Wohland T, Eggeling C, Ozhan G. Binding of canonical Wnt ligands to their receptor complexes occurs in ordered plasma membrane environments. FEBS J. 2017 Aug; 284(15): 2513-2526. doi: 10.1111/febs.14139.
Akyerli C., Yuksel S., Can O., Erson-Omay Z., Oktay Y.*, Cosgun E., Ulgen E., Erdemgil Y., San A., von Deimling A., Gunel M., Yakicier C., Pamir M.N., Ozduman K. Use of telomerase promoter mutations to mark specific molecular subsets with reciprocal clinical behavior in IDH mutant and IDH wild-type diffuse gliomas. Journal of Neurosurgery. 2017 Jun; 16:1-13. doi: 10.3171/2016.11.
Schneider F, Waithe D, Clausen MP, Galiani S, Koller T, Ozhan G, Eggeling C, Sezgin E. Diffusion of lipids and GPI-anchored proteins in actin-free plasma membrane vesicles measured by STED-FCS. Mol Biol Cell. 2017 Jun 1;28(11): 1507-1518. doi: 10.1091/mbc.E16-07-0536.
Oktay Y., Boylu C.A., Özduman K. Gliom Gelişiminde Genetik Yatkınlığın Rolü. Türk Nöroşirürji Dergisi. 2017 May; 27(2): 122-130.
Gökhan Karakülah, Aslı Suner. PlanTEnrichment: A tool for enrichment analysis of transposable elements in plants. Genomics. 2017 (in press), doi:10.1016/j.ygeno.2017.05.008.
Bağırsakçı E., Şahin E., Atabey N., Erdal E. & Carr B.I. Role of Albumin in growth inhibition in Hepatocellular Carcinoma. Oncology. 2017 May 10. doi:10.1159/000471807.
Gümürdü A., Yildiz R., Eren E., Karakülah G., Ünver T., Genç Ş. & Park Y. MicroRNA exocytosis by large dense-core vesicle fusion. Scientific Reports. 2017 Mar 30; 7:45661. doi:10.1038/srep45661.
Marino IR, Carr B.I. New Developments in Orthotopic Liver Transplant for Hepatocellular Carcinoma. Exp Clin Transplant. 2017 Mar;15(Suppl 2):1-6.
Alural B, Genc S, Haggarty S. Diagnostic and therapeutic potential of microRNAs in neuropsychiatric disorders: Past, present, and future. Prog Neuropsychopharmacol Biology Psychiatry. 2017 Feb; 6;73:87-103 doi: 10.1016/j.pnpbp.2016.03.010. Epub 2016 Apr 9. PubMed PMID: 27072377; PubMed Central PMCID: PMC5292013.
Pinato D., Sharma R., Allara E., Yen C., Arizumi T., Kubota K., Bettinger D., Jang J.W., Smirne C., Kim Y.W., Kudo M., Howell J., Ramaswami R., Burlone M.E., Guerra V., Thimme R., Ishizuka M., Stebbing J., Pirisi M. & Carr B.I. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J. Hepatology, 2017 Feb; 66(2): 338-346. doi:http://dx.doi.org/10.1016/j.jhep.2016.09.008.
van Waalwijk van Doorn LJ, Gispert JD, Kuiperij HB, et al. Improved Cerebrospinal Fluid-Based Discrimination between Alzheimer’s Disease Patients and Controls after Correction for Ventricular Volumes. J Alzheimers Dis. 2017 Jan 24; 56(2):543-555. doi: 10.3233/JAD-160668
Park CG, Park Y & Suh BC. The HOOK region of voltage-gated Ca2+ channel β subunits senses and transmits PIP2 signals to the gate. J Gen Physiol. 2017 Jan 13. doi:10.1085/jgp.201611677.
Arslan N, Guzel O, Kose E, Yılmaz U, Kuyum P, Aksoy B, Çalık T. Is ketogenic diet treatment hepatotoxic for children with intractable epilepsy? Seizure. 2016 Dec; 43: 32-38. doi:10.1016/j.seizure.2016.10.024.
Ebru Diler, Turgay Unver & Gökhan Karakülah. Differential Expression of Hyperhydricity Responsive Peach miRNAs. Journal of Integrative Bioinformatics. 2016 Dec; 13(5): 308. doi:10.2390/biecoll-jib-2016-308.
İşcan E, Güneş A, Korhan P, Yılmaz Y, Erdal E, Atabey N. The regulatory role of heparin on c-Met signaling in hepatocellular carcinoma cells. J Cell Commun Signal. 2016 Dec 14; 1-12. doi:10.1007/s12079-016-0368-0.
Gökhan Karakülah, Kuaybe Yücebilgili Kurtoğlu, Turgay Unver. PeTMbase: A Database of Plant Endogenous Target Mimics (eTMs). PLoS ONE. 2016 Dec 9; 11(12): e0167698. doi:10.1371/journal.pone.0167698.
Calibasi Kocal G, Güven S, Foygel K, Goldman A, Chen P, Sengupta S, Paulmurugan R, Baskin Y, Demirci U. Dynamic Microenvironment Induces Phenotypic Plasticity of Esophageal Cancer Cells Under Flow. Sci Rep. 2016 Dec 2; 6:38221. doi: 10.1038/srep38221.
Jung-Woong Kim, Hyun-Jin Yang, Matthew John Brooks, Lina Zelinger, Gökhan Karakülah*, Norimoto Gotoh, Alexis Boleda, Linn Gieser, Felipe Giuste, Dustin Thad Whitaker, Ashley Walton, Rafael Villasmil, Jennifer Joanna Barb, Peter Jonathan Munson, Koray Dogan Kaya, Vijender Chaitankar, Tiziana Cogliati, Anand Swaroop. NRL-Regulated Transcriptome Dynamics of Developing Rod Photoreceptors. Cell Reports. 2016 Nov 22; 17(9): 2460-2473. doi:http://dx.doi.org/10.1016/j.celrep.2016.10.074.
Arslan N, Kose E, Guzel O. The Effect of Ketogenic Diet on Serum Selenium Levels in Patients with Intractable Epilepsy. Biol Trace Elem Res. 2016 Nov 21. doi:10.1007/s12011-016-0897-7.
Erbayraktar Z, Alural B, Erbayraktar RS, Erkan EP. Cell division cycle 7-kinase inhibitor PHA-767491 hydrochloride suppresses glioblastoma growth and invasiveness. Cancer Cell Int. 2016 Nov 18;16:88. eCollection 2016. doi:10.1186/s12935-016-0364-8.
Muhammad Ayaz Mustufa, Cigdem Ozen, Imran Ali Hashmi, Afshan Aslam, Jameel Ahmed Baig, Gokhan Yildiz, Shoaib Muhammad, Imam Bakhsh Solangi, Naim ul Hasan Naqvi, Mehmet Ozturk and Firdous Imran Ali. Synthesis and bio-molecular study of (+)-N-Acetyl-α-amino acid dehydroabietylamine derivative for the selective therapy of hepatocellular carcinoma. BMC Cancer. 2016, Nov 14; 16: 883. doi:10.1186/s12885-016-2942-5.
Büyüköz M, Erdal E, Alsoy Altinkaya S. Nanofibrous gelatin scaffolds integrated with NGF-loaded alginate microspheres for brain tissue engineering. J Tissue Eng Regen Med. 2016 Nov 12. doi: 10.1002/term.2353.
Jeannot V, Busser B, Vanwonterghem L, Michallet S, Ferroudj S, Cokol M, Coll JL, Ozturk M, Hurbin A. Synergistic activity of vorinostat combined with gefitinib but not with sorafenib in mutant KRAS human non-small cell lung cancers and hepatocarcinoma. OncoTargets and Terapy. 2016, Nov 9; 9: 6843—6855. doi:10.2147/OTT.S117743.
Carr B.I., Guerra V., Giannini E.G. et al. A Liver Index and its Relationship to Indices of HCC Aggressiveness. J Integr Oncol. 2016 Oct;5(4). pii: 178. doi: 10.4172/2329-6771.1000178.
Erkan D, Kayali HA. Replacement of Soybean Meal with Animal Origin Protein Meals Improved Ramoplanin A2 Production by Actinoplanes sp. ATCC 33076. Appl Biochem Biotechnol. 2016 Sep;180(2):306-21. doi:10.1007/s12010-016-2100-1.
Hani Alotaibi, Nese Atabey, Kasım Diril, Esra Erdal, Mehmet Ozturk. Molecular Mechanisms of Hepatocellular Carcinoma, Chapter Hepatocellular Carcinoma Part of the series Current Clinical Oncology. Springer. 2016, Aug 27; 43-63. doi:10.1007/978-3-319-34214-6_3.
Alagoz Y., Gurkok T., Zhang B. & Unver T. Manipulating the Biosynthesis of Bioactive Compound Alkaloids for Next-Generation Metabolic Engineering in Opium Poppy Using CRISPR-Cas 9 Genome Editing Technology. Sci Rep. 2016 Aug 3; 6:30910. doi: 10.1038/srep30910.
Bakır Y., Eldem V., Zararsız G. & Unver T. Global Transcriptome Analysis Reveals Differences in Gene Expression Patterns Between Nonhyperhydric and Hyperhydric Peach Leaves. Plant Genome. 2016 Jul; 9(2). doi:10.3835/plantgenome2015.09.0080.
Marsano A, Medeiros da Cunha CM, Ghanaati S, Gueven S, Centola M, Tsaryk R, Barbeck M, Stuedle C, Barbero A, Helmrich U, Schaeren S, Kirkpatrick JC, Banfi A, Martin I. Spontaneous In Vivo Chondrogenesis of Bone Marrow-Derived Mesenchymal Progenitor Cells by Blocking Vascular Endothelial Growth Factor Signaling. Stem Cells Transl Med. 2016 Jul 26. doi: 10.5966/sctm.2015-0321.
Pavlopoulou A., Oktay Y., Vougas K., Louka M., Vorgias C.E., Georgakilas A.G. Determinants of resistance to chemotherapy and ionizing radiation in breast cancer stem cells. Cancer Letters, 2016, Jul 19; 380(2):485-493. doi: 10.1016/j.canlet.2016.07.018.
Carr B.I., Guerra V., Giannini E.O. et al. An HCC Aggressiveness Index and Blood GTP, Blirubin and Platelet Levels. J. Integrative Oncology. 2016 Jun 20; 5:172. doi: 10.4172/2329-6771.1000172.
Oktay Y., Ülgen E., Can Ö., Akyerli C.B., Yüksel Ş., Erdemgil Y., Durası I.M., Henegariu O.I., Nanni E.P., Selevsek N., Grossmann J., Erson-Omay E.Z., Bai H., Gupta M., Lee W., Turcan Ş., Özpınar A., Huse J.T., Sav M.A., Flanagan A., Günel M., Sezerman O.U., Yakıcıer M.C., Pamir M.N. & Özduman K. IDH-mutant glioma specific association of rs55705857 located at 8q24.21 involves MYC deregulation. Sci Rep. 2016 Jun 10; 6: 27569. doi: 10.1038/srep27569.
Yilmaz Y, Atabey N, Erdal E & Brian I. Carr. Platelets, Microenvironment and Hepatocellular Carcinoma. Biochemistry & Analytical Biochemistry, 2016 June 29; 5:281 (Review). doi:10.4172/2161-1009.1000281.
Antonio Mazzoccaa, Giovanni Ferrarob, Giovanni Misciagnac, Brian I. Carr. A systemic evolutionary approach to cancer: Hepatocarcinogenesis as a paradigm. Medical Hypotheses, 2016, Aug; 93: 132-137. doi:10.1016/j.mehy.2016.05.027.
Karakülah G, Karakuş M, Suner A, Demir S, Arserim SK, Töz S, Özbel Y. sandflyDST: a dynamic web-based decision support tool for the morphological identification of sandflies present in Anatolia and mainland Europe, and user study. Medical and Veterinary Entomology, 2016 June 24. doi:10.1111/mve.12182.
Can Küçük, Xiaozhou Hu, Qiang Gong, Bei Jiang, Adam Cornish, Philippe Gaulard, Timothy McKeithan, Wing C. Chan. Diagnostic and Biological Significance of KIR Expression Profile Determined by RNA-Seq in Natural Killer/T-Cell Lymphoma. The American Journal of Pathology, 2016 June; 186(6): 1435-1441. doi:10.1016/j.ajpath.2016.02.011.
Zeynep Firtina Karagonlar, Doğukan Koç, Eren Şahin, Sanem Tercan Avci, Mustafa Yilmaz, Neşe Atabey, Esra Erdal. Effect of adipocyte-secreted factors on EpCAM+/CD133+ hepatic stem cell population. Biochemical and Biophysical Research Communications, 2016 June 3; 474(3): 482-490. doi:10.1016/j.bbrc.2016.04.137.
Yakup Bakır, Vahap Eldem, Gökmen Zararsız, Turgay Unver. Global Transcriptome Analysis Reveals Significant Differences in Gene Expression Patterns Between The Non-Hyperhydric and Hyperhydric Leaves of Prunus persica. The Plant Genome, 2016, May; 9(2): 1-9.
Salimi R, Yener N, Safari R. Use and Evaluation of Newly Synthesized Fluorescence Probes to Detect Generated OH• Radicals in Fibroblast Cells. J Fluoresc. 2016 May; 26(3):919-24. doi: 10.1007/s10895-016-1780-9.
Firtina Karagonlar Z, Koc D, Iscan E, Erdal E, Atabey N. Elevated hepatocyte growth factor expression as an autocrine c‐Met activation mechanism in acquired resistance to sorafenib in hepatocellular carcinoma cells. Cancer Sci. 2016 Apr; 107(4):407-416 doi: 10.1111/cas.12891.
Carr BI & Guerra V. A Hepatocellular Carcinoma Aggressiveness Index and Its Relationship to Liver Enzyme Levels. Oncology 2016 Mar 15; 90(4). doi: 10.1159/000444394.
Gao LM, Zhao S, Liu WP, Zhang WY, Li GD, Küçük C, Hu XZ, Chan WC, Tang Y, Ding WS, Yan JQ, Yao WQ, Wang JC. Clinicopathologic Characterization of Aggressive Natural Killer Cell Leukemia Involving Different Tissue Sites. Am J Surg Pathol, 2016 Mar 11. doi: 10.1097/PAS.0000000000000634.
Carr BI & Guerra V. Low Alpha-Fetoprotein Levels Are Associated with Improved Survival in Hepatocellular Carcinoma Patients with Portal Vein Thrombosis. Digestive Diseases and Sciences, 2016 Feb 26; 61(3):937-947. doi: 10.1007/s10620-015-3922-3.
Ferroudj S, Yildiz G, Bouras M, Iscan E, Ekin U, Ozturk M. Role of fanconi anemia/BRCA pathway genes in hepatocellular carcinoma chemoresistance. Hepatol Res. 2016 Feb 16. doi: 10.1111/hepr.12675.
Carr BI & Guerra V. Hepatocellular Carcinoma Extrahepatic Metastasis in Relation to Tumor size and Alkaline Phosphatase Levels. Oncology 2016 Feb 12; 90(3):1-7. doi: 10.1159/000443480.
Durmaz I, Guven EB, Ersahin T, Ozturk M, Calis I, Cetin-Atalay R. Liver cancer cells are sensitive to Lanatoside C induced cell death independent of their PTEN status. Phytomedicine. 2016 Jan 15; 23(1):42-51. doi: 10.1016/j.phymed.2015.11.012.
Wingender G. From the deep sea to everywhere? Environmental antigens for iNKT cells. Arch Immunol Ther Exp, 2015 Dec 24 (Review). doi: 10.1007/s00005-015-0381-7.
Güzel O, Yılmaz U, Uysal U, Arslan N. The effect of olive oil-based ketogenic diet on serum lipid levels in epileptic children. Neurol Sci. 2015 Dec 23; 1-6. doi: 10.1007/s10072-015-2436-2.
Namkoong B, Guven S, Ramesan S, Liaudanskaya V, Abzhanov A, Demirci U. Recapitulating cranial osteogenesis with neural crest cells in 3-D microenvironments. Acta Biomaterialia, 2015 Dec 07. doi: 10.1016/j.actbio.2015.12.004.
Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, Herrley E, Rasquinha N, McArdle S, Wu R, Peluso E, Metzger D, Ichinose H, Shaked I, Chodaczek G, Biswas SK, Hedrick CC. Patrolling Monocytes Control Tumor Metastasis to the Lung. Science. 2015, Nov 20; 350(6263): 985-90.
Wingender G & Kronenberg M. Characterization of human T cell subsets via surface markers. Cytometry A, 2015, Oct 27; 87A; 1067-1069.
D’Alessandro R, Messa C, Refolo MG, Carr BI. Modulation of sensitivity and resistance to multikinase inhibitors by microenvironmental platelet factors in HCC. Expert Opinion on Pharmacotherapy. 2015 Oct 19; 16(18):1-8. doi: 10.1517/14656566.2015.1101065.
Wingender G, Sag D, Kronenberg M. NKT10 cells: a novel iNKT cell subset. Oncotarget. 2015 Sept 29; 6(29): 26552-26553 (Review). doi: 10.18632/oncotarget.5270.
Wingender G, Birkholz A, Sag D, Farber E, Chitale S, Howell AR, Kronenberg M. Selective conditions are required for the induction of iNKT cell hypo-responsiveness by antigenic stimulation. The Journal of Immunology. 2015 Sept 9; 195: 3838-3848. doi: 10.4049/jimmunol.1500203.
Lippolis C, Refolo MG, D’Alessandro R, Carella N, Messa C, Cavallini A, Carr BI. Resistance to multikinase inhibitor actions mediated by insulin like growth factor-1. Journal of Experimental & Clinical Cancer Research. 2015 Sept 2; 34(1):90. doi: 10.1186/s13046-015-0210-1.
Pančoška P, Skála L, Nešetřil J, Carr BI. Validation of the Concept of a Common Typical Time of Disease Duration for Hepatocellular Carcinoma Patients Using the Fisher Information Processing of Tumor Imaging Results Combined With Network Phenotyping Strategy Quantification of Individual Patient Clinical Profile Patterns. Seminars in Oncology. 2015 Aug; 42(4):672-678. doi: 10.1053/j.seminoncol.2015.05.004.
Gunes A, Iscan E, Topel H, Avci ST, Gumustekin M, Erdal E, Atabey N. Heparin treatment increases thioredoxin interacting protein expression in hepatocellular carcinoma cells. The International Journal of Biochemistry & Cell Biology. 2015 Aug; 65:169-181. doi: 10.1016/j.biocel.2015.05.025.
Hu X, Chan WC, Kücük C. Generation of a genetically engineered aggressive nk-cell leukemia cell line with stable IL2 expression. Acta Medica International. 2015 Jul; 2(2):78-84. doi: 10.5530/ami.2015.3.6.
Ozhan G and Weidinger G. Wnt/β-catenin signaling in heart regeneration. Cell Regen (Lond). 2015 Jul 8; 4(1):3. doi: 10.1186/s13619-015-0017-8
Alotaibi H, Basilicata MF, Shehwana H, Kosowan T, Schreck I, Braeutigam C, Konu O, Brabletz T, Stemmler MP. Enhancer cooperativity as a novel mechanism underlying the transcriptional regulation of E-cadherin during mesenchymal to epithelial transition. Biochimica et Biophysica Acta (BBA) – Gene Regulatory Mechanisms. 2015 June; 1849(6):731-742. doi: 10.1016/j.bbagrm.2015.01.005.
Alural B, Ozerdem A, Allmer J, Genc K, Genc S. Lithium protects against paraquat neurotoxicity by NRF2 activation and miR-34a inhibition in SH-SY5Y cells. Front Cell Neurosci. 2015 May 28; 9:209. doi: 10.3389/fncel.2015.00209.
Dilek Cevik, Gokhan Yıldız & Mehmet Ozturk. Common telomerase reverse transcriptase promoter mutations in hepatocellular carcinomas from different geographical locations. World J Gastroenterol. 2015 Jan 7; 21(1): 311–317. doi: 10.3748/wjg.v21.i1.311.
Alural B, Duran GA, Tufekci KU, Allmer J, Onkal Z, Tunali D, Genc K, Genc S. EPO Mediates Neurotrophic, Neuroprotective, Anti-Oxidant, and Anti-Apoptotic Effects via Downregulation of miR-451 and miR-885-5p in SH-SY5Y Neuron-Like Cells. Frontiers in Immunology. 2014 Sep 30; 5:475. doi: 10.3389/fimmu.2014.00475.
* Current address IBG.
| Core Manager Çiğdem Özen, PhD, Specialist |
The Hybridoma and Monoclonal Antibody Production Core Facility will provide service for researchers and biotechnology companies. The aim is to establish an efficient and affordable production of antibodies and other biological materials. Our service covers the whole process from antigen immunization in mice, rat or rabbit to production of purified monoclonal antibodies. We use hybridoma technology that includes generation of hybrid cell lines or hybridomas by fusing an antibodyproducing B cell with an immortal myeloma cell. The facility produces both monoclonal and polyclonal antibodies and provides antibody purification service using different benchtop chromatography techniques. Following purification of the antibody, the IgG type can also be characterized on request. Furthermore, the facility offers consultation service on all aspects of monoclonal antibody production.
| Coordinator | |
| Ralph Meuwissen, PhD | |
| Members | |
| Kasım Diril, PhD | Duygu Sağ, PhD |
| Ensari Güneli, DVM, PhD | Gerhard Wingender, PhD |
| Güneş Özhan, PhD | |
SCOPE
Advanced animal models of human diseases continue to expand their use for both descriptive basic research as well as applied preclinical research. In this program, we focus on preclinical research with a clear emphasis on functional drug screening and validation. Our ultimate goal will be the translation of these preclinical results on disease intervention into clinical trials. For this, we will use existing animal models and where appropriate; we will design novel, better-suited models. In vitro drug screen assays typically rely on simple interactions of chemicals with a drug target, such as receptor binding or enzyme activity inhibition. However, in vitro results often poorly correlate with in vivo results because of the complicated physiological environment is absent in the in vitro testing system. Although cell-based assays can provide some information, cultured cells still do not provide physiological conditions and complex interactions among different cell types and tissues. Therefore, results in animal studies are essential to validate high – throughput screening (HTS) hits and exclude compounds with unfavorable absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties, which are responsible for more than half of compound attrition in costly clinical trials. Currently, in vivo assays are not usually performed until or after the lead optimization stage. This is partly due to the low speed and high cost of conventional animal models (typically rodents) and the relatively high number of preliminary hits from HTS. With alternative small-animal models, however, it is now possible to perform in vivo testing earlier in the development process. Thus, researchers have developed systems using both vertebrates (e.g. zebrafish) and invertebrates (e.g. the fruit fly Drosophila melanogaster) for drug screening. The small size, high fecundity, and experimental tractability of these animals enable costeffective and rapid screening of numerous compounds. The experimental animal facility at iBG-izmir maintains and utilizes the whole breath of animal model systems, namely: Drosophila, zebrafish and rodents (mice/rats)
FOCUS AREAS AND MILESTONES
The emphasis of each model system will be:
• Drosophila: Drosophila is being used frequently in high impact studies to model, for example, cancer and diabetes, because of the simple ability to perform genome-wide modifier and drug compound screens. Simple phenotypes associated with human mutations in conserved disease orthologs can be examined. We will establish novel animal models of diseases in the Drosophila system, to uncover genetic disease mechanism involving loss and gain of known pathway function. Furthermore, we will screen for pathway modifications by genetic and pharmacological modifiers. To best complement our work on wound healing, high priority will be placed on exploring the interactions between epithelial healing mechanisms and metabolic disorders, such as diabetes.
• Zebrafish: Wnt/β-catenin signaling pathway components are considered as preferred targets for drug development as malfunctioning of the pathway is associated with human diseases including cancer. Unfortunately, targeted treatment of the underlying cause of these diseases is currently not possible, since very few compounds are known to specifically inhibit β-catenin signaling. In the coming years, we aim to perform a drug screen for Wnt-related cancers using zebrafish, which has become an attractive model for high-throughput drug screening. Zebrafish embryos offer a unique system where pharmacological molecules can directly be added to the embryo water and become readily absorbed. They allow for direct observation of phenotypic hallmarks of cancer, such as enhanced cell proliferation and angiogenesis, reduced apoptosis, abnormal activation of oncogenes, repression of tumor suppressor genes and changes in cell migratory behavior. Compounds can be identified via embryonic screens and further validated in adults for their molecular mechanisms to pinpoint them as a target for a specific cancer type. For this purpose, we aim to exploit zebrafish as a drug discovery model and a biopharmaceutical service by collaborating with pharmaceutical companies to identify novel drug candidates. Another strength of the zebrafish system is that it represents an exclusive example of a highly regenerative vertebrate model, due to its distinct regenerative capacity in various organs and tissues including the heart, pancreas, liver, muscle, skin, blood and pigment cells, fins and the CNS. Here, we aim to establish part of the zebrafish facility as a unit that can serve to collaborate for the regeneration-related research projects of internal and external laboratories that would like to benefit from this unique regeneration model.
• Rodents (rats/mice): At iBG-izmir we will make use of well-established mouse models of cancer and metastasis (mainly liver and lung cancer), diabetes, obesity, experimental allergic encephalomyelitis (EAE), viral and bacterial infections, using wild-type and humanized mice. Some congenic rat strains for diabetes and obesity research will also be used. However, genetically modified mice strains will be the most suitable models for e.g. cancer and some other diseases. Our fully integrated transgenic core facility will not only enable us to adapt existing models but also to design new ones. Such advanced genetically modified mice will make use of state-of-the-art technologies, including Cre/lox, Flp/frt conditional alleles or Tet/on-off inducible transgenes. In this way we, will have accurate somatic mouse models for diseases that are ideally suited to test drug candidates for targeted therapy against defined molecular pathway(s). As described above, mice models are not practical for HTS of drugs, but rather will be used for preclinical therapy testing. For example, mice strains that express luciferase and fluorescent protein reporter alleles will enable us to follow disease interventions in real time by non-invasive in vivo imaging at our core facility. We will establish and maintain the essential number of genetically modified mice strains for the various diseases of interest. The core will also become a member of the European consortium for Mouse Phenotyping and Archiving Research Infrastructure (INFRAFRONTIER) to become the central facility for Turkey. Thereby, we will be access and participate in the large non-profit genetically modified mice repository, the European Mouse Mutant Archive (EMMA). These cooperations will greatly enhance the interaction with our European partners and our access to funding.
• Lastly, we will set up Patient Derived Xenotransplant (PDX) models for lung cancer. These PDX models recapitulate the heterogeneity of human cancer, to a much higher extend than conventional mouse models do. Recent experiments with severe immune compromised NOD-SCID-Gamma (NSG) recipient mice have shown very promising results in maintaining human primary tumor tissue characteristics after transplantation. PDX models are thus a very important supplement to our preclinical drug testing capacity. Here too we initiated participation efforts to become member of the EUROPDX consortium for mutual use and exchange of PDX cancer models between European partner institutes.
• Development and testing of drug HTS in both Drosphila and zebrafish systems. Here our focus will be on small molecule drug testing. Our minimal goals are the development of one or more candidates during the first 3 years, and the validation of at least one molecule after preclinical testing to allow its use for follow-up clinical trials. The funding will be obtained from TUBITAK and industrial partners.
• Development and optimization of genetically modified mice models for lung and liver cancer, as well as of PDX models for lung cancer. These models will be used to test preclinical and targeted be specific monoclonal antibodies, small molecules, or biosimilars. Our minimal goals during the first 3 years are the establishment and functional characterization of the mouse models, together with conclusive validation results for the candidate drug. Our mouse model should then be ready as a tool for preclinical testing to make candidate drugs ready for clinical trials. Funding will be obtained from TUBİTAK, European consortia (Infrafrontier), EU research funds and industrial partners.
| Coordinator | |
| Sinan Güven, PhD | |
| Members | |
| Neşe Atabey, PhD | Ralph Meuwissen, PhD |
| Can Küçük, PhD | Yavuz Oktay, PhD |
SCOPE
Medical diagnostics is the key element for providing the right therapeutic approach to curing diseases. Finding, describing and correlating the chemical and physical symptoms of diseases demands devices equipped with highly precise and accurate sensors. Modern clinical approaches utilize different methods for diagnostics such as using specific biomarkers, hi-tech imaging systems, genomic analysis methods, high-throughput screening and point of care technologies. Bio-microdevices are miniaturized technologically engineered platforms that provide high and precise control over environmental variables and mimic the native physiological conditions of living organisms. Biosensor-supplied microdevices are excellent tools for diagnostics, as they minimize the sample volumes, analysis time and cost of tests. In the Program for Diagnostics and Medical Microdevices at iBG-izmir, we formed a multidisciplinary research team of bioengineers, molecular biologists, physicists, chemists and clinicians to develop micro- and nano-devices for diagnostics. With close clinical collaborations, we will implement the most current analytical techniques (such as next generation sequencing) and design biosensors for detection of diagnostic markers from patient samples (such as body fluids, circulating tumor cells or solid tissues). Cancer, infectious agents, metabolic, autoimmune and neurological diseases are in the scope of the program. For these we aim to generate reliable, rapid and cost effective micro- and nano-diagnostic devices. The minimal success criterion of the program is to bring an innovative, micro-/nanodiagnostic device on the medical market. iBG-izmir and this Innovation Program will advance the medical R&D activities in the region and will be a key component providing competitive products to the healthcare market. Furthermore, Dokuz Eylul University also hosts the BioIzmir initiative and DEPARK (Dokuz Eylul Technology Development Zone), which both focus on the industrial translation of medical innovations. The cooperation with these organizations will further increase the impact of this Innovation Program.
The program is going to obtain substantial funds from TÜBITAK, EU research funds, NIH partnerships, private and industrial funding.
FOCUS AREAS AND MILESTONES
• Develop and validate at least one reliable, rapid, low cost, easy to operate and cost-efficient diagnostic device.
• Apply for a patent of the developed device and its technology.
• Validation and commercialization of the device.
| Coordinator | |
| Yavuz Oktay, PhD | |
| Members | |
| Nur Arslan, MD | Semra Hız Kurul, MD |
| Can Küçük, PhD | |
SCOPE
The incidence of many rare diseases is much higher in Turkey due to the high rate of relative (consanguineous) marriages. The clinical presentation of such rare diseases is highly heterogeneous, and therefore the proper diagnosis and treatment of these diseases is currently challenging. Additionally, the genetic background of most of these diseases is poorly defined. However, the identification of the underlying genetic aberrations will likely lead to the development of better diagnostic, prognostic and therapeutic strategies. Recently, the discovery of nextgeneration sequencing (NGS)-based methodologies rendered it possible to identify disease-associated mutations with a genome-wide scope. The work within this program will apply whole-exome sequencing (WES) to identify specific mutations associated with rare diseases, with the aim to generate unique diagnostic panels. Moreover, we will identify driver mutations with the potential to function as novel therapeutic targets. Specifically, we plan to focus on rare diseases of the nervous system, such as epilepsy syndromes and neurodegenerative diseases.
FOCUS AREAS AND MILESTONES
5 year goals:
• Establish at least one multi-disciplinary consortium that involves pathologists, clinicians, genome and bioinformatics scientists.
• To collect and store all relevant rare disease-specific clinical and histopathological data in a central database for at least three rare disease types.
• To generate a NGS-based disease panel for at least two rare disease groups. Funding options: TUBITAK (1001, 1003, 1007 grant programs), Izmir Agency of Development (IZKA), and EU funding programs (Horizon 2020 etc).
Success criteria (3rd and 5th years):
• Exome and/or targeted sequencing and bioinformatic analysis of at least 300 patients within three years and of at least 500 patients within five years.
• Identification and validation of all mutations necessary for proper diagnosis in at least one rare disease within three years and of at least two rare disease within five years.
• Development of at least 2 NGS panels for 2 different rare diseases (5th year).
| Coordinator | |
| Şermin Genç, MD, PhD | |
| Members | |
| Kasım Diril, PhD | Gerhard Wingender, PhD |
| Sinan Güven, PhD | Yavuz Oktay, PhD |
SCOPE
Neurological disorders include Alzheimer disease and other dementias, stroke, multiple sclerosis, Parkinson’s disease, brain tumors, traumatic disorders of the nervous system such as brain trauma, epilepsy and migraine. Hundreds of millions of people worldwide are affected by neurological disorders. For example, stroke is the third most common cause of death worldwide. Alzheimer’s disease is the most common type of dementia, affecting 35 million individuals in the world, with an expected threefold increase by 2050. Furthermore, multiple sclerosis, an inflammatory demyelinating disease of the brain and the spinal cord, affects two million people globally, with rates varying widely in different regions and populations. However, many aspects of etiology, genetics and pathogenesis of neurological disorders are still not clearly defined. In addition, for many of these diseases, no good predictive or diagnostic test is available. Finally, and most importantly, for many neurological disorders the current treatments are largely symptomatic and no final cure can be achieved. For these reasons, neurological disorders have become a major health problem for many countries, whose burden is expected to rise with the aging of the population. Turkey’s Science and Technology High Council declared ‘Neuroscience’ as one of the priority areas of health and technology. The Neurological Disorders Program at iBG-izmir aims to identify novel genes/mutations responsible for familial and non-familial neurological disorders, to discover new biomarkers and to develop novel therapeutic tools and approaches for the treatment of these disorders. To this end, we will study a wide range of patient samples, as well as established and newly generated in vitro and in vivo animal models of neurological disorders. The funding will be obtained from TUBITAK, Ministry of Health, industrial partners and EU research funds.
FOCUS AREAS AND MILESTONES
The focus areas and 5 years goals with milestones of the Program are as follows:
• Development of in vitro and in vivo models: 3D cultures of induced pluripotent stem cells to study differentiation
to neural stem cells, current type neural or glial cells. In addition to in vitro models, we aim to develop new transgenic animal models of neurological disorders. Our minimal goals are the development of one novel in vitro or in vivo model during the first 3 years, and another one within 5 years.
• Discovery of novel predictive genes/mutations: Many neurological disorders have a genetic etiology. We will investigate familial and non-familial forms of neurological disorders to find disease causing novel genes/mutations by whole exome sequencing. Our minimal goals are biobanking of at least 30 families during the first 3 years, and whole exome sequencing within 5 years.
• Biomarker discovery and development of diagnostic tools: Circulating serum micro RNAs (miRNAs), and other possible biomarkers will be investigated to find novel biomarkers for neurological novel mutations will be developed. Additionally, these biomarkers and novel driver mutations in neurological cancers could be possible targets for treatment. Our minimal goals are to identify one novel biomarker or target molecule during the first 3 years, and finish preclinical validation within 5 years.
| Coordinator | |
| Gerhard Wingender, PhD | |
| Members | |
| Duygu Sağ, PhD |
SCOPE
Immunotherapy is the treatment of diseases via the alteration of the immune system. This can be achieved by inducing, enhancing or suppressing immune responses, depending on the context. Current approaches in immunotherapy are heavily biased to the adaptive immune system by targeting or utilizing conventional T and B cells. However, the innate immune system, and innate like T cells are known to be crucially involved in most, if not all, inflammatory responses. This is particular relevant for chronic inflammatory diseases. Nonetheless, only few immunotherapies based on the innate and innate-like immune system are currently under development. Recognizing the great potential for novel and innovative therapeutic approaches, this program focuses on these aspects, with a particular emphasis on macrophages and innate like T cells.
FOCUS AREAS AND MILESTONES
• Macrophage polarization as therapeutic target: Pro-inflammatory macrophages, which promote inflammation, are called M1 macrophages. In contrast, anti-inflammatory macrophages, which decrease inflammation and support tissue repair, are called M2 macrophages. How environmental factors influence this M1/M2 – balance is poorly defined. Recent intriguing findings by one program member demonstrated that the activity of a cholesterol transporter strongly influences this M1/M2 – balance. This altered macrophage balance had profound impact on tumor immunity. By linking cholesterol transporters to tumor immunity, this finding provides an unprecedented link between the metabolism and chronic inflammatory diseases, including cancer. The intermediate 3 years goal of this project is to find and characterize small molecules and/or monoclonal antibodies to modulate the activity of cholesterol transporters. The long-term 5 years goal is to utilize these reagents as therapeutic approach to alter macrophage polarization for cancer immunotherapy and potentially for metabolic/inflammatory diseases.
• iNKT cell based cell therapy: Innate T cells are a unique subset of T cells that combine features of innate NK cells and of adaptive memory T cells. The most prominent member of innate T cells are invariant Natural Killer (iNKT) cells. Following antigenic stimulation, iNKT cells rapidlyproduce copious amounts of various cytokines and thereby can have a pronounced effect on the immune system. They are known to impact an impressive variety of different immune reactions, ranging from chronic and acute inflammatory processes, including responses to pathogens and tumors, to autoimmune responses. As all humans share basically identical iNKT cells, their therapeutic potential is great. Recently, program members characterized a novel iNKT cell subset with potent IL-10-dependent regulatory function, which were termed NKT10 cells. These, NKT10 cells could impair anti-tumor immune responses and protect mice against experimental autoimmune encephalomyelitis (EAE), a
mouse model of multiple sclerosis. The intermediate 3 years goal of this project is to better characterize the regulatory mechanisms of NKT10 cells and to optimize their expansion in vitro and in vivo. The long-term 5 years goal is to utilize NKT10 cells as cell therapy for EAE and for obesity related metabolic diseases.
For both projects funding by one major national or international grant should be obtained within 3 years. We expect for both projects to acquire proof-of-concept preclinical in vivo data in wild type and humanized animals within a 5 years period.
| Coordinator | |
| Esra Erdal, PhD | |
| Members | |
| Nur Arslan, MD, PhD | Sinan Güven, PhD |
| Neşe Atabey, PhD | Güneş Özhan, PhD |
SCOPE
Regenerative medicine is one of the most promising and rapidly advancing areas of modern medicine, as it focuses on innovative approaches to repair and replace cells, tissues and organs. Cell therapies implement patient oriented approaches, and provide novel venues for personalized medicine. At iBG-izmir, we will establish an internationally accredited cGMP facility for the production of therapeutic grade cells and tissues for humans. Our program brings together basic research scientists and clinicians focused on utilizing cells and stem cells in the reconstruction of tissues and organs, as well as engineering cells for therapeutic approaches. Cell therapies and regenerative medicine have already been applied in clinics as experimental and alternative methods. With this program we aim to develop and translate cell-based approaches as validated standard treatment method for clinicians. In this regard, we focus on basic and translational medical sciences utilizing cell therapies, design clinical trials and set new therapeutic standards. Achieving the goals of the program is strongly coupled with innovative thinking and creating value in therapeutic medicine.
FOCUS AREAS AND MILESTONES
• Developing cell based pre-clinical in vitro and in vivo disease models underlining the mechanisms of regeneration.
• Generating autologous and allogeneic adult mesenchymal stem cells from bone marrow, adipose tissue and dental pulp; or from specialized cells, like limbal stem cells, chondrocytes and hepatocytes. Implementing their use for cancer, trauma, plastic and reconstructive surgery, ophthalmology and dental applications.
• Developing and translating cell based therapy methods from Bench-to-Bedside in accordance with national and international guidelines.
• Design and start a clinical trial for cell-based therapy. Funding of the program will be obtained from TUBITAK, Ministry of Health, EU research funds and the Dokuz Eylul University Hospital.
| Coordinator Mehmet Öztürk, PhD |
|
| Vice Coordinator Şerif Şentürk, PhD |
|
| Members | |
| Hani Alotaibi, PhD | Kasım Diril, PhD |
| Neşe Atabey, PhD | Ensari Güneli, DVM, PhD |
| Hülya Ayar Kayalı, PhD | Ralph Meuwissen, PhD |
SCOPE
Targeted therapeutics are drugs or other substances such as antibodies acting on specific molecules (“molecular targets”) that are involved in the pathogenesis or complications of a disease. Targeted therapeutics are also called “precision medicines”. Most of targeted therapeutics approved by FDA or EMEA are used against cancer, but their field of application is rapidly spreading to other diseases such as autoimmune and rare diseases. The process of targeted therapeutics development usually starts by the discovery of a critical molecular target, followed by the choice of appropriate targeting molecules, in vitro and in vivo validation studies, production under cGMP conditions and clinical trials.
Many of the currently available targeted therapeutics are “biopharmaceuticals” with expired patents. Consequently, there is a growing interest in Turkey and other countries in the manufacturing of their generic forms called “biosimilars” as they are more affordable. Considering global trends and local needs, iBG-izmir decided to invest its scientific and technological means to cover a broad area of application starting from production of biosimilars and leading towards the development of innovative biopharmaceuticals. Investigation of innovative targeted drugs such as small chemical inhibitors will also be covered by the program. Accordingly, iBG-izmir is building a GMP facility accompanied by quality control, animal toxicology and pharmacology units, dedicated to biosimilar production, as well as state-of-the-art “drug discovery” facilities.
FOCUS AREAS AND MILESTONES
• Development and manufacturing of biosimilars: humanized monoclonal antibodies (Mabs) and antibodylike molecules are the initial focus area. Our minimal goals are the development of one of more biosimilar master cell banks during the first 3 years, and the production of at least one biosimilar molecule ready for initial clinical trials. The funding will be obtained from TUBITAK and industrial partners.
• Development of “biobetter” and innovative Mabs: A “biobetter Mab” is a novel antibody acting on a known target by a different mechanism for example via a different epitope. Several projects on anti-cancer Mab development against validated and novel target molecules will be launched. Our minimal goals are the development of one of more humanized Mab master cell banks during the first 3 years, and preclinical validation within 5 years. The funding will be obtained from TUBITAK, EU research funds and industrial partners.
• Development and validation generic small molecule inhibitors for combined therapy against refractory or relapsed cancer. Patient-derived (chemo)therapy naïve primary tumors together with their refractory or relapsed tumors can be used to screen anti-cancer small molecules efficacy against validated and novel targets from activated molecular pathways. Our minimal goals are the collection and availability of distinct sets of generic small molecules that will be tested in (primary) tumor cultures. This will be followed within 5 years by preclinical validation in our patient-derived-xenotransplant (PDX) models and our advanced, genetically modified animal models.
Funding will be obtained through TUBITAK, EU framework collaborations and industrial partners.

By loading the map, you agree to Google's privacy policy.
Learn more
| İzmir Uluslararası Biyotıp ve Genom Enstitüsü (iBG-izmir) Dokuz Eylül Üniversitesi Sağlık Yerleşkesi Balçova 35340 İzmir |
Telefon: 0(232) 412 86 51 Faks: 0(232) 412 63 53 E-posta: ibg@deu.edu.tr |
Enstitü Sekreterliği
| Enstitü Sekreteri | Mukaddes AKKEÇELİ | +90 232 412 86 54 | mukaddes.akkeceli@deu.edu.tr |
| Özel Kalem | Özlem ALKU | +90 232 412 86 51 | ozlem.alku@deu.edu.tr |
İdari Birimler
| Öğrenci İşleri | Gamze Güven | +90 232 412 86 66 | gamze.guven@deu.edu.tr |
| Yazı İşleri ve Evrak Kayıt Birimi | Özlem Alku | +90 232 412 86 70 | ozlem.alku@deu.edu.tr |
| Personel İşleri/Bölüm Sekreterliği | Naciye Yıldız | +90 232 412 86 63 | naciye.yildiz@deu.edu.tr |
| Dış İlişkiler Birimi | Özlem Alku | +90 232 412 86 51 | ozlem.alku@deu.edu.tr |
| İdari ve Mali İşler Birimi | Haktan Güner | +90 232 412 86 61 | haktan.guner@deu.edu.tr |
| Taşınır Kayıt ve Kontrol Birimi | Haktan Güner | +90 232 412 86 61 | haktan.guner@deu.edu.tr |

By loading the map, you agree to Google's privacy policy.
Learn more
| Izmir International Biomedicine and Genome Institute (iBG-izmir) Dokuz Eylul Universitesi Saglik Yerleskesi Balcova 35340 Izmir/TURKEY |
Phone: +90(232) 412 67 04 Fax: +90(232) 277 63 53 E-mail: ibg@deu.edu.tr |
| İzmir Uluslararası Biyotıp ve Genom Enstitüsü (iBG-izmir) Dokuz Eylül Üniversitesi Sağlık Yerleşkesi Balçova 35340 İzmir |
Telefon: +90(232) 412 67 04 Faks: +90(232) 277 63 53 E-posta: ibg-izmir@deu.edu.tr |
2024/2025 Öğretim Yılı Güz Yarıyılı Ders Programı MBG BST
2024/2025 Güz Yarıyılı 2. Başvuru Giriş Sınavında Başarılı Olan Öğrenciler
2024/2025 Öğretim Yılı Güz Yarıyılı 2. Başvuru Linki
2024/25 Eğitim-Öğretim Yılı Güz Yarıyılı 2. Dönem Öğrenci Alımı İlan Metni
2024/2025 Güz Yarıyılı Giriş Sınavında Başarılı Olan Öğrenciler
2024/2025 Eğitim-Öğretim Yılı Güz Yarıyılı İlan Metni
2023/2024 Öğretim Yılı Bahar Yarıyılı Moleküler Biyoloji ve Genetik Anabilim Dalı Ders Programı 2023/2024 Öğretim Yılı Bahar Yarıyılı Biyotıp ve…
2024/2025 Güz Yarıyılı 2. Başvuru Giriş Sınavında Başarılı Olan Öğrenciler
2024/2025 Öğretim Yılı Güz Yarıyılı 2. Başvuru Linki
2024/25 Eğitim-Öğretim Yılı Güz Yarıyılı 2. Dönem Öğrenci Alımı İlan Metni
2024/2025 Güz Yarıyılı Giriş Sınavında Başarılı Olan Öğrenciler
2024/2025 Eğitim-Öğretim Yılı Güz Yarıyılı İlan Metni
2023/2024 Öğretim Yılı Bahar Yarıyılı Moleküler Biyoloji ve Genetik Anabilim Dalı Ders Programı 2023/2024 Öğretim Yılı Bahar Yarıyılı Biyotıp ve…
2023/2024 Öğretim Yılı Bahar Yarıyılı Kayıt Yenileme Web Kayıt Takvimi